Molecular Diversity of Midbrain Development in Mouse, Human, and Stem Cells
- PMID: 27716510
- PMCID: PMC5055122
- DOI: 10.1016/j.cell.2016.09.027
Molecular Diversity of Midbrain Development in Mouse, Human, and Stem Cells
Abstract
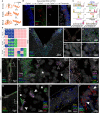
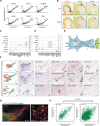
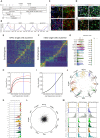
Understanding human embryonic ventral midbrain is of major interest for Parkinson's disease. However, the cell types, their gene expression dynamics, and their relationship to commonly used rodent models remain to be defined. We performed single-cell RNA sequencing to examine ventral midbrain development in human and mouse. We found 25 molecularly defined human cell types, including five subtypes of radial glia-like cells and four progenitors. In the mouse, two mature fetal dopaminergic neuron subtypes diversified into five adult classes during postnatal development. Cell types and gene expression were generally conserved across species, but with clear differences in cell proliferation, developmental timing, and dopaminergic neuron development. Additionally, we developed a method to quantitatively assess the fidelity of dopaminergic neurons derived from human pluripotent stem cells, at a single-cell level. Thus, our study provides insight into the molecular programs controlling human midbrain development and provides a foundation for the development of cell replacement therapies.
Keywords: dopaminergic neuron; human; mouse; single-cell RNA-seq; ventral midbrain.
Copyright © 2016 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures












References
-
- Acampora D., Mazan S., Lallemand Y., Avantaggiato V., Maury M., Simeone A., Brûlet P. Forebrain and midbrain regions are deleted in Otx2-/- mutants due to a defective anterior neuroectoderm specification during gastrulation. Development. 1995;121:3279–3290. - PubMed
-
- Almqvist P.M., Åkesson E., Wahlberg L.U., Pschera H., Seiger A., Sundström E. First trimester development of the human nigrostriatal dopamine system. Exp. Neurol. 1996;139:227–237. - PubMed
-
- Andersson E., Tryggvason U., Deng Q., Friling S., Alekseenko Z., Robert B., Perlmann T., Ericson J. Identification of intrinsic determinants of midbrain dopamine neurons. Cell. 2006;124:393–405. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

