A Phase I Dose Escalation Study of the Triple Angiokinase Inhibitor Nintedanib Combined with Low-Dose Cytarabine in Elderly Patients with Acute Myeloid Leukemia
- PMID: 27716819
- PMCID: PMC5055288
- DOI: 10.1371/journal.pone.0164499
A Phase I Dose Escalation Study of the Triple Angiokinase Inhibitor Nintedanib Combined with Low-Dose Cytarabine in Elderly Patients with Acute Myeloid Leukemia
Abstract
Nintedanib (BIBF 1120), a potent multikinase inhibitor of VEGFR-1/-2/-3, FGFR-1/-2/-3 and PDGFR-α/-β, exerts growth inhibitory and pro-apoptotic effects in myeloid leukemic cells, especially when used in combination with cytarabine. This phase I study evaluated nintedanib in combination with low-dose cytarabine (LDAC) in elderly patients with untreated or relapsed/refractory acute myeloid leukemia (AML) ineligible for intensive chemotherapy in a 3+3 design. Nintedanib (dose levels 100, 150, and 200 mg orally twice daily) and LDAC (20 mg subcutaneous injection twice daily for 10 days) were administered in 28-day cycles. Dose-limiting toxicity (DLT) was defined as non-hematological severe adverse reaction CTC grade ≥ 4 with possible or definite relationship to nintedanib. Between April 2012 and October 2013, 13 patients (median age 73 [range: 62-86] years) were enrolled. One patient did not receive study medication and was replaced. Nine (69%) patients had relapsed or refractory disease and 6 (46%) patients had unfavorable cytogenetics. The most frequently reported treatment-related adverse events (AE) were gastrointestinal events. Twelve SAEs irrespective of relatedness were reported. Two SUSARs were observed, one fatal hypercalcemia and one fatal gastrointestinal infection. Two patients (17%) with relapsed AML achieved a complete remission (one CR, one CRi) and bone marrow blast reductions without fulfilling PR criteria were observed in 3 patients (25%). One-year overall survival was 33%. Nintedanib combined with LDAC shows an adequate safety profile and survival data are promising in a difficult-to-treat patient population. Continuation of this trial with a phase II recommended dose of 2 x 200 mg nintedanib in a randomized, placebo-controlled phase II study is planned. The trial is registered to EudraCT as 2011-001086-41.
Trial registration: ClinicalTrials.gov NCT01488344.
Conflict of interest statement
This study was funded by Boehringer Ingelheim Pharma GmbH & Co. KG, which does not alter the authors' adherence to PLOS ONE policies on sharing data and materials. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Figures
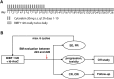
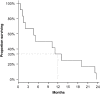
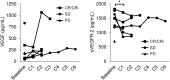
References
-
- Buchner T, Berdel WE, Haferlach C, Haferlach T, Schnittger S, Muller-Tidow C, et al. Age-related risk profile and chemotherapy dose response in acute myeloid leukemia: a study by the German Acute Myeloid Leukemia Cooperative Group. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27(1):61–9. Epub 2008/12/03. 10.1200/JCO.2007.15.4245 . - DOI - PubMed
-
- Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–74. Epub 2009/11/03. 10.1182/blood-2009-07-235358 . - DOI - PubMed
-
- Buchner T, Berdel WE, Schoch C, Haferlach T, Serve HL, Kienast J, et al. Double induction containing either two courses or one course of high-dose cytarabine plus mitoxantrone and postremission therapy by either autologous stem-cell transplantation or by prolonged maintenance for acute myeloid leukemia. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006;24(16):2480–9. Epub 2006/06/01. 10.1200/JCO.2005.04.5013 . - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

