Signaling Properties of Chemerin Receptors CMKLR1, GPR1 and CCRL2
- PMID: 27716822
- PMCID: PMC5055294
- DOI: 10.1371/journal.pone.0164179
Signaling Properties of Chemerin Receptors CMKLR1, GPR1 and CCRL2
Abstract
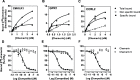
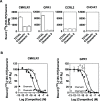
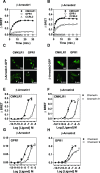
Chemerin is a small chemotactic protein originally identified as the natural ligand of CMKLR1. More recently, two other receptors, GPR1 and CCRL2, have been reported to bind chemerin but their functional relevance remains poorly understood. In this study, we compared the binding and signaling properties of the three human chemerin receptors and showed differences in mode of chemerin binding and receptor signaling. Chemerin binds to all three receptors with low nanomolar affinities. However, the contribution of the chemerin C-terminus to binding efficiency varies greatly amongst receptors. By using BRET-based biosensors monitoring the activation of various G proteins, we showed that binding of chemerin and the chemerin 9 nonapeptide (149YFPGQFAFS157) to CMKLR1 activates the three Gαi subtypes (Gαi1, Gαi2 and Gαi3) and the two Gαo isoforms (Gαoa and Gαob) with potencies correlated to binding affinities. In contrast, no significant activation of G proteins was detected upon binding of chemerin to GPR1 or CCRL2. Binding of chemerin and the chemerin 9 peptide also induced the recruitment of β-arrestin1 and 2 to CMKLR1 and GPR1, though to various degree, but not to CCRL2. However, the propensity of chemerin 9 to activate β-arrestins relative to chemerin is higher when bound to GPR1. Finally, we showed that binding of chemerin to CMKLR1 and GPR1 promotes also the internalization of the two receptors and the phosphorylation of ERK1/2 MAP kinases, although with a different efficiency, and that phosphorylation of ERK1/2 requires both Gαi/o and β-arrestin2 activation but not β-arrestin1. Collectively, these data support a model in which each chemerin receptor displays selective signaling properties.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures








References
-
- Zabel BA, Silverio AM, Butcher EC (2005) Chemokine-like receptor 1 expression and chemerin-directed chemotaxis distinguish plasmacytoid from myeloid dendritic cells in human blood. J Immunol 174: 244–251. 174/1/244 [pii]. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

