Paracetamol (acetaminophen) for prevention or treatment of pain in newborns
- PMID: 27716943
- PMCID: PMC6611486
- DOI: 10.1002/14651858.CD011219.pub3
Paracetamol (acetaminophen) for prevention or treatment of pain in newborns
Update in
-
Paracetamol (acetaminophen) for prevention or treatment of pain in newborns.Cochrane Database Syst Rev. 2020 Jan 27;1(1):CD011219. doi: 10.1002/14651858.CD011219.pub4. Cochrane Database Syst Rev. 2020. PMID: 31985830 Free PMC article.
Abstract
Background: Newborn infants have the ability to experience pain. Hospitalised infants are exposed to numerous painful procedures. Healthy newborns are exposed to pain if the birth process consists of assisted vaginal birth by vacuum extraction or by forceps and during blood sampling for newborn screening tests.
Objectives: To determine the efficacy and safety of paracetamol for the prevention or treatment of procedural/postoperative pain or pain associated with clinical conditions in neonates. To review the effects of various doses and routes of administration (enteral, intravenous or rectal) of paracetamol for the prevention or treatment of pain in neonates.
Search methods: We used the standard search strategy of the Cochrane Neonatal Review group to search the Cochrane Central Register of Controlled Trials (CENTRAL 2016, Issue 4), MEDLINE via PubMed (1966 to 9 May 2016), Embase (1980 to 9 May 2016), and CINAHL (1982 to 9 May 2016). We searched clinical trials' databases, Google Scholar, conference proceedings, and the reference lists of retrieved articles.
Selection criteria: We included randomised and quasi-randomised controlled trials of paracetamol for the prevention/treatment of pain in neonates (≤ 28 days of age).
Data collection and analysis: Two review authors independently extracted data from the articles using pre-designed forms. We used this form to decide trial inclusion/exclusion, to extract data from eligible trials and to request additional published information from authors of the original reports. We entered and cross-checked data using RevMan 5 software. When noted, we resolved differences by mutual discussion and consensus. We used the GRADE approach to assess the quality of evidence.
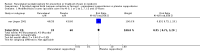
Main results: We included nine trials with low risk of bias, which assessed paracetamol for the treatment of pain in 728 infants. Painful procedures studied included heel lance, assisted vaginal birth, eye examination for retinopathy of prematurity assessment and postoperative care. Results of individual studies could not be combined in meta-analyses as the painful conditions, the use of paracetamol and comparison interventions and the outcome measures differed. Paracetamol compared with water, cherry elixir or EMLA cream (eutectic mixture of lidocaine and prilocaine) did not significantly reduce pain following heel lance. The Premature Infant Pain Profile score (PIPP) within three minutes following lancing was higher in the paracetamol group than in the oral glucose group (mean difference (MD) 2.21, 95% confidence interval (CI) 0.72 to 3.70; one study, 38 infants). Paracetamol did not reduce "modified facies scores" after assisted vaginal birth (one study, 119 infants). In another study (n = 123), the Échelle de Douleur et d'Inconfort du Nouveau-Né score at two hours of age was significantly higher in the group that received paracetamol suppositories than in the placebo suppositories group (MD 1.00, 95% CI 0.60 to 1.40). In that study, when infants were subjected to a heel lance at two to three days of age, Bernese Pain Scale for Neonates scores were higher in the paracetamol group than in the placebo group, and infants spent a longer time crying (MD 19 seconds, 95% CI 14 to 24). For eye examinations, no significant reduction in PIPP scores in the first or last 45 seconds of eye examination was reported, nor at five minutes after the eye examination. In one study (n = 81), the PIPP score was significantly higher in the paracetamol group than in the 24% sucrose group (MD 3.90, 95% CI 2.92 to 4.88). In one study (n = 114) the PIPP score during eye examination was significantly lower in the paracetamol group than in the water group (MD -2.70, 95% CI -3.55 to 1.85). For postoperative care following major surgery, the total amount of morphine (µg/kg) administered over 48 hours was significantly less among infants assigned to the paracetamol group than to the morphine group (MD -157 µg/kg, 95% CI -27 to -288). No adverse events were noted in any study. The quality of evidence according to GRADE was low.
Authors' conclusions: The paucity and low quality of existing data do not provide sufficient evidence to establish the role of paracetamol in reducing the effects of painful procedures in neonates. Paracetamol given after assisted vaginal birth may increase the response to later painful exposures. Paracetamol may reduce the total need for morphine following major surgery, and for this aspect of paracetamol use, further research is needed.
Conflict of interest statement
Dr Arne Ohlsson is a co‐author of one of the included trials (Shah 1998).
Dr Prakeshkumar Shah reports no conflicts of interest.
Figures
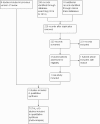


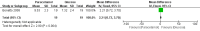






































Update of
-
Paracetamol (acetaminophen) for prevention or treatment of pain in newborns.Cochrane Database Syst Rev. 2015 Jun 25;(6):CD011219. doi: 10.1002/14651858.CD011219.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2016 Oct 07;10:CD011219. doi: 10.1002/14651858.CD011219.pub3. PMID: 26110914 Updated.
Comment in
-
In newborns, oral or rectal paracetamol fails to reduce procedural pain, whereas intravenous paracetamol reduces morphine requirements after major surgery.Evid Based Med. 2016 Jun;21(3):93. doi: 10.1136/ebmed-2016-110400. Epub 2016 Feb 24. Evid Based Med. 2016. PMID: 26912573 No abstract available.
References
References to studies included in this review
-
- Badiee Z, Torcan N. Effects of high dose orally administered paracetamol for heel prick pain in premature infants. Saudi Medical Journal 2009;30(11):1450‐3. [; PUBMED: 19882059] - PubMed
-
2799577
-
- Bonetto G, Salvatico E, Varela N, Cometto C, Gómez PF, Calvo B. Pain prevention in term neonates: randomized trial for three methods [Prevención del dolor en recién nacidos de término: estudio aleatorizado sobre tres métodos]. Archivos Argentinos de Pediatría 2008;106(5):392‐6. [; PUBMED: 19030637] - PubMed
-
2799579
-
- Ceelie I, Wildt SN, Dijk M, Berg MM, Bosch GE, Duivenvoorden HJ, et al. Effect of intravenous paracetamol on postoperative morphine requirements in neonates and infants undergoing major noncardiac surgery: a randomized controlled trial. JAMA 2013;309(2):149‐54. [; PUBMED: 23299606] - PubMed
-
2799581
-
- Kabataş EU, Dursun A, Beken S, Dilli D, Zenciroğlu A, Okumuş N. Efficacy of single dose oral paracetamol in reducing pain during examination for retinopathy of prematurity: A blinded randomized controlled trial. Indian Journal of Pediatrics 2016;83(1):22‐6. [] - PubMed
-
4291670
-
- Manjunatha CM, Ibhanesebhor SE, Rennix C, Fisher H, Abara R. Pain control during retinopathy of prematurity screening: double‐blind, randomised, placebo‐controlled study. Infant 2009;5(5):155‐8. []
-
2799583
References to studies excluded from this review
-
- Härmä A, Aikio O, Hallman M, Saarela T. Intravenous paracetamol decreases requirements of morphine in very preterm Infants. Journal of Pediatrics 2016;168:36‐40. [] - PubMed
-
4291672
-
- Hong JY, Kim WO, Koo BN, Cho JS, Suk EH, Kil HK. Fentanyl‐sparing effect of acetaminophen as a mixture of fentanyl in intravenous parent‐/nurse‐controlled analgesia after pediatric ureteroneocystostomy. Anesthesiology 2010;113(3):672–7. [; PUBMED: 20693884] - PubMed
-
2799597
References to studies awaiting assessment
-
- Foronda O, Rocha S, Grandy G. Efficacies of paracetamol and glucose in pain reduction in newborns: A randomized, controlled and single blind study. Pediatric Research. 2014; Vol. 75, issue 3:473. []
-
4291674
-
- Garbi LR, Shah S. The effect of intravenous acetaminophen on opioid use in preterm infants. Pediatric Academic societies (PAS) Annual Meeting; 2016 Apr 30 ‐ May 3; Baltimore, USA. 2016:362. []
-
4291676
-
- Marel CD, Peters JW, Bouwmeester NJ, Jacqz‐Aigrain E, Anker JN, Tibboel D. Rectal acetaminophen does not reduce morphine consumption after major surgery in young infants. British Journal of Anaesthesia 2007;98(3):372‐9. [; PUBMED: 17284514] - PubMed
-
2799599
References to ongoing studies
-
- NCT01938261. The preterm infants' paracetamol study (PreParaS). clinicaltrials.gov/show/NCT01938261. ClinicalTrials.gov, (accessed 13 October 2013). []
-
2799601
Additional references
-
- American Academy of Pediatrics, Committee on Fetus and Newborn, Committee on Drugs, Section on Anesthesiology, Section on Surgery. Neonatal anesthesia. Pediatrics 1987;80:446. - PubMed
-
- American Academy of Pediatrics, Committee on Fetus and Newborn, Committee on Drugs, Section on Anesthesiology, Section on Surgery, Canadian Paediatric Society Fetus and Newborn Committee. Prevention and management of pain and stress in the neonate. Pediatrics 2000;105(2):454‐61. [PUBMED: 10654977] - PubMed
-
- American Academy of Pediatrics Committee on Fetus and Newborn, Section on Surgery, Canadian Paediatric Society Fetus and Newborn Committee, Batton DG, Barrington KJ, Wallman C. Prevention and management of pain in the neonate: an update. Pediatrics 2006;118(5):2231‐41. [PUBMED: 17079598] - PubMed
-
- Ambuel B, Hamlett KW, Marx CM, Blumer JL. Assessing distress in pediatric intensive care environments: the COMFORT scale. Journal of Pediatric Psychology 1992;17(1):95‐109. [PUBMED: 1545324] - PubMed
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th Edition. Arlington, VA: American Psychiatric Association, 2013.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

