Specifically neuropathic Gaucher's mutations accelerate cognitive decline in Parkinson's
- PMID: 27717005
- PMCID: PMC5244667
- DOI: 10.1002/ana.24781
Specifically neuropathic Gaucher's mutations accelerate cognitive decline in Parkinson's
Abstract
Objective: We hypothesized that specific mutations in the β-glucocerebrosidase gene (GBA) causing neuropathic Gaucher's disease (GD) in homozygotes lead to aggressive cognitive decline in heterozygous Parkinson's disease (PD) patients, whereas non-neuropathic GD mutations confer intermediate progression rates.
Methods: A total of 2,304 patients with PD and 20,868 longitudinal visits for up to 12.8 years (median, 4.1) from seven cohorts were analyzed. Differential effects of four types of genetic variation in GBA on longitudinal cognitive decline were evaluated using mixed random and fixed effects and Cox proportional hazards models.
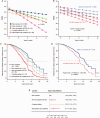
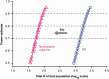
Results: Overall, 10.3% of patients with PD and GBA sequencing carried a mutation. Carriers of neuropathic GD mutations (1.4% of patients) had hazard ratios (HRs) for global cognitive impairment of 3.17 (95% confidence interval [CI], 1.60-6.25) and a hastened decline in Mini-Mental State Exam scores compared to noncarriers (p = 0.0009). Carriers of complex GBA alleles (0.7%) had an HR of 3.22 (95% CI, 1.18-8.73; p = 0.022). By contrast, the common, non-neuropathic N370S mutation (1.5% of patients; HR, 1.96; 95% CI, 0.92-4.18) or nonpathogenic risk variants (6.6% of patients; HR, 1.36; 95% CI, 0.89-2.05) did not reach significance.
Interpretation: Mutations in the GBA gene pathogenic for neuropathic GD and complex alleles shift longitudinal cognitive decline in PD into "high gear." These findings suggest a relationship between specific types of GBA mutations and aggressive cognitive decline and have direct implications for improving the design of clinical trials. Ann Neurol 2016;80:674-685.
© 2016 The Authors. Annals of Neurology published by Wiley Periodicals, Inc. on behalf of American Neurological Association.
Figures

Comment in
-
Glucocerebrosidase, Parkinson disease, and the "senses and intellect".Ann Neurol. 2016 Nov;80(5):660-661. doi: 10.1002/ana.24808. Ann Neurol. 2016. PMID: 27779773 No abstract available.
References
-
- Hruska KS, LaMarca ME, Scott CR, Sidransky E. Gaucher disease: mutation and polymorphism spectrum in the glucocerebrosidase gene (GBA). Hum Mutat 2008;29:567–583. - PubMed
-
- Grabowski GA, Zimran A, Ida H. Gaucher disease types 1 and 3: phenotypic characterization of large populations from the ICGG Gaucher Registry. Am J Hematol 2015;90(suppl 1):S12–S18. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

