Effects of Ethanol on Cellular Composition and Network Excitability of Human Pluripotent Stem Cell-Derived Neurons
- PMID: 27717039
- PMCID: PMC5391051
- DOI: 10.1111/acer.13218
Effects of Ethanol on Cellular Composition and Network Excitability of Human Pluripotent Stem Cell-Derived Neurons
Abstract
Background: Prenatal alcohol exposure (PAE) in animal models results in excitatory-inhibitory (E/I) imbalance in neocortex due to alterations in the GABAergic interneuron (IN) differentiation and migration. Thus, E/I imbalance is a potential cause for intellectual disability in individuals with fetal alcohol spectrum disorder (FASD), but whether ethanol (EtOH) changes glutamatergic and GABAergic IN specification during human development remains unknown. Here, we created a human cellular model of PAE/FASD and tested the hypothesis that EtOH exposure during differentiation of human pluripotent stem cell-derived neurons (hPSNs) would cause the aberrant production of glutamatergic and GABAergic neurons, resulting in E/I imbalance.
Methods: We applied 50 mM EtOH daily to differentiating hPSNs for 50 days to model chronic first-trimester exposure. We used quantitative polymerase chain reaction, immunocytochemical, and electrophysiological analysis to examine the effects of EtOH on hPSN specification and functional E/I balance.
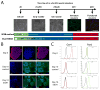
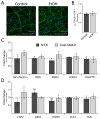
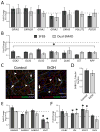
Results: We found that EtOH did not alter neural induction nor general forebrain patterning and had no effect on the expression of markers of excitatory cortical pyramidal neurons. In contrast, our data revealed highly significant changes to levels of transcripts involved with IN precursor development (e.g., GSX2, DLX1/2/5/6, NR2F2) as well as mature IN specification (e.g., SST, NPY). Interestingly, EtOH did not affect the number of GABAergic neurons generated nor the frequency or amplitude of miniature excitatory and inhibitory postsynaptic currents.
Conclusions: Similar to in vivo rodent studies, EtOH significantly and specifically altered the expression of genes involved with IN specification from hPSNs, but did not cause imbalances of synaptic excitation-inhibition. Thus, our findings corroborate previous studies pointing to aberrant neuronal differentiation as an underlying mechanism of intellectual disability in FASD. However, in contrast to rodent binge models, our chronic exposure model suggests possible compensatory mechanisms that may cause more subtle defects of network processing rather than gross alterations in total E/I balance.
Keywords: Cortical Neurons; Excitatory-Inhibitory Balance; Fetal Alcohol Spectrum Disorder; Interneurons; Somatostatin.
Copyright © 2016 by the Research Society on Alcoholism.
Conflict of interest statement
Disclosure of Potential Conflicts of Interest: The authors indicate no potential conflict of interest.
Figures
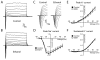

References
-
- Ayoola AB, Stommel M, Nettleman MD. Late recognition of pregnancy as a predictor of adverse birth outcomes. American journal of obstetrics and gynecology. 2009;201:156, e151–156. - PubMed
-
- Bell SH, Stade B, Reynolds JN, Rasmussen C, Andrew G, Hwang PA, Carlen PL. The remarkably high prevalence of epilepsy and seizure history in fetal alcohol spectrum disorders. Alcoholism, clinical and experimental research. 2010;34:1084–1089. - PubMed
-
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nature reviews Neuroscience. 2002;3:728–739. - PubMed
-
- Borodinsky LN, Root CM, Cronin JA, Sann SB, Gu X, Spitzer NC. Activity-dependent homeostatic specification of transmitter expression in embryonic neurons. Nature. 2004;429:523–530. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

