Effect of GLP-1 receptor agonist treatment on body weight in obese antipsychotic-treated patients with schizophrenia: a randomized, placebo-controlled trial
- PMID: 27717222
- PMCID: PMC5299524
- DOI: 10.1111/dom.12795
Effect of GLP-1 receptor agonist treatment on body weight in obese antipsychotic-treated patients with schizophrenia: a randomized, placebo-controlled trial
Erratum in
-
Erratum: Effect of GLP-1 receptor agonist treatment on body weight in obese antipsychotic-treated patients with schizophrenia: A randomized, placebo-controlled trial.Diabetes Obes Metab. 2018 May;20(5):1327-1328. doi: 10.1111/dom.13204. Epub 2018 Jan 23. Diabetes Obes Metab. 2018. PMID: 29316175 Free PMC article. No abstract available.
Abstract
Aims: Schizophrenia is associated with cardiovascular co-morbidity and a reduced life-expectancy of up to 20 years. Antipsychotics are dopamine D2 receptor antagonists and are the standard of medical care in schizophrenia, but the drugs are associated with severe metabolic side effects such as obesity and diabetes. Glucagon-like peptide-1 receptor agonists (GLP-1RAs) are registered for treatment of both obesity and type 2 diabetes. We investigated metabolic effects of the GLP-1RA, exenatide once-weekly, in non-diabetic, antipsychotic-treated, obese patients with schizophrenia.
Material and methods: Antipsychotic-treated, obese, non-diabetic, schizophrenia spectrum patients were randomized to double-blinded adjunctive treatment with once-weekly subcutaneous exenatide (n = 23) or placebo (n = 22) injections for 3 months. The primary outcome was loss of body weight after treatment and repeated measures analysis of variance was used as statistical analysis.
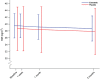
Results: Between March 2013 and June 2015, 40 patients completed the trial. At baseline, mean body weight was 118.3 ± 16.0 kg in the exenatide group and 111.7 ± 18.0 kg in the placebo group, with no group differences ( P = .23). The exenatide and placebo groups experienced significant ( P = .004), however similar ( P = .98), weight losses of 2.24 ± 3.3 and 2.23 ± 4.4 kg, respectively, after 3 months of treatment.
Conclusions: Treatment with exenatide once-weekly did not promote weight loss in obese, antipsychotic-treated patients with schizophrenia compared to placebo. Our results could suggest that the body weight-lowering effect of GLP-1RAs involves dopaminergic signaling, but blockade of other receptor systems may also play a role. Nevertheless, anti-obesity regimens effective in the general population may not be readily implemented in antipsychotic-treated patients with schizophrenia.
Trial registration: ClinicalTrials.gov NCT01794429.
Keywords: GLP-1 analogue; antidiabetic drug; exenatide; randomized trial schizophrenia.
© 2016 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Figures
References
-
- McGrath J, Saha S, Chant D, Welham J. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev. 2008;30(1):67‐76. - PubMed
-
- Saha S, Chant D, Mcgrath J. A systematic review of mortality in schizophrenia. Arch Gen Psychiatry. 2007;64(10):1123‐1131. - PubMed
-
- Baskin ML, Ard J, Franklin F, Allison DB. Prevalence of obesity in the United States. Obes Rev. 2005;6(1):5‐7. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

