Comparative Effectiveness of Newer Medications for Lower Urinary Tract Symptoms Attributed to Benign Prostatic Hyperplasia: A Systematic Review and Meta-analysis
- PMID: 27717522
- PMCID: PMC5337128
- DOI: 10.1016/j.eururo.2016.09.032
Comparative Effectiveness of Newer Medications for Lower Urinary Tract Symptoms Attributed to Benign Prostatic Hyperplasia: A Systematic Review and Meta-analysis
Abstract
Context: Alpha-blockers (ABs) and 5-alpha reductase inhibitors have an established role in treating male lower urinary tract symptoms (LUTS) attributed to benign prostatic hyperplasia (BPH). Recently, newer drugs have shown promise for this indication.
Objective: To assess the comparative effectiveness and adverse effects (AEs) of newer drugs to treat LUTS attributed to BPH through a systematic review and meta-analysis.
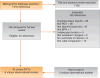
Evidence acquisition: Ovid MEDLINE, the Cochrane Central Register of Controlled Trials, and Ovid Embase bibliographic databases (through June 2016) were hand searches for references of relevant studies. Eligible studies included randomized controlled trials published in English of newer ABs, antimuscarinics, a beta-3 adrenoceptor agonist, phosphodiesterase type-5 inhibitors, or combination therapy with one of these medications as an active comparator. Observational studies of the same agents with a duration ≥1 yr that reported AEs were also included.
Evidence synthesis: We synthesized evidence from 43 randomized controlled trials as well as five observational studies. Based on improvement of mean International Prostate Symptom Score and quality of life scores, the effectiveness of the newer ABs was not different from the older ABs (moderate strength of evidence [SOE]), but had more AEs (low SOE). Antimuscarinics/AB combination therapy had similar outcomes as AB monotherapy (all moderate SOE), but often had more AEs. Phosphodiesterase type-5 inhibitors alone or in combination with ABs had similar or inferior outcomes than ABs alone. Evidence was insufficient for the beta-3 adrenoceptor agonist. For all newer agents, the evidence was generally insufficient to assess long-term efficacy, prevention of symptom progression, or AEs.
Conclusions: None of the drugs or drug combinations newly used to treat LUTS attributed to BPH showed outcomes superior to traditional AB treatment. Given the lack of superior outcomes, the studies' short time-horizon, and less assurance of their safety, their current value in treating LUTS attributable to BPH appears low.
Patient summary: In this paper, we reviewed the evidence of newer drugs to treat men with urinary problems attributable to an enlarged prostate. We found none of the new drugs to be better but there was more concern about side effects.
Keywords: 5-alpha reductase inhibitor; Alpha blockers; Benign prostatic hyperplasia; Comparative effectiveness; Lower urinary tract symptoms; Randomized trials; Systematic review.
Copyright © 2016 European Association of Urology. All rights reserved.
Conflict of interest statement
Figures
Comment in
-
Medical Treatment of Male Lower Urinary Tract Symptoms: Does One Fit All?Eur Urol. 2017 Apr;71(4):582-583. doi: 10.1016/j.eururo.2016.11.019. Epub 2016 Nov 30. Eur Urol. 2017. PMID: 27914901 No abstract available.
References
-
- MacDonald R, Wilt TJ. Alfuzosin for treatment of lower urinary tract symptoms compatible with benign prostatic hyperplasia: a systematic review of efficacy and adverse effects. Urology. 2005;66:780–8. - PubMed
-
- MacDonald R, Wilt TJ, Howe RW. Doxazosin for treating lower urinary tract symptoms compatible with benign prostatic obstruction: a systematic review of efficacy and adverse effects. BJU Int. 2004;94:1263–70. - PubMed
-
- Wilt TJ, Howe W, MacDonald R. Terazosin for treating symptomatic benign prostatic obstruction: a systematic review of efficacy and adverse effects. BJU Int. 2002;89:214–25. - PubMed
-
- Wilt TJ, MacDonald R, Nelson D. Tamsulosin for treating lower urinary tract symptoms compatible with benign prostatic obstruction: a systematic review of efficacy and adverse effects. J Urol. 2002;167:177–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

