Trafficking of adeno-associated virus vectors across a model of the blood-brain barrier; a comparative study of transcytosis and transduction using primary human brain endothelial cells
- PMID: 27718541
- PMCID: PMC5298820
- DOI: 10.1111/jnc.13861
Trafficking of adeno-associated virus vectors across a model of the blood-brain barrier; a comparative study of transcytosis and transduction using primary human brain endothelial cells
Abstract
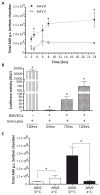
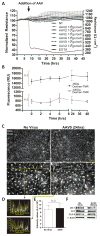
Developing therapies for central nervous system (CNS) diseases is exceedingly difficult because of the blood-brain barrier (BBB). Notably, emerging technologies may provide promising new options for the treatment of CNS disorders. Adeno-associated virus serotype 9 (AAV9) has been shown to transduce cells in the CNS following intravascular administration in rodents, cats, pigs, and non-human primates. These results suggest that AAV9 is capable of crossing the BBB. However, mechanisms that govern AAV9 transendothelial trafficking at the BBB remain unknown. Furthermore, possibilities that AAV9 may transduce brain endothelial cells or affect BBB integrity still require investigation. Using primary human brain microvascular endothelial cells as a model of the human BBB, we performed transduction and transendothelial trafficking assays comparing AAV9 to AAV2, a serotype that does not cross the BBB or transduce endothelial cells effectively in vivo. Results of our in vitro studies indicate that AAV9 penetrates brain microvascular endothelial cells barriers more effectively than AAV2, but has reduced transduction efficiency. In addition, our data suggest that (i) AAV9 penetrates endothelial barriers through an active, cell-mediated process, and (ii) AAV9 fails to disrupt indicators of BBB integrity such as transendothelial electrical resistance, tight junction protein expression/localization, and inflammatory activation status. Overall, this report shows how human brain endothelial cells configured in BBB models can be utilized for evaluating transendothelial movement and transduction kinetics of various AAV capsids. Importantly, the use of a human in vitro BBB model can provide import insight into the possible effects that candidate AVV gene therapy vectors may have on the status of BBB integrity. Read the Editorial Highlight for this article on page 192.
Keywords: adeno-associated virus; blood-brain barrier; gene therapy; neurological disorders.
© 2016 International Society for Neurochemistry.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nature reviews. Neuroscience. 2006;7:41–53. - PubMed
-
- Banks WA, Akerstrom V, Kastin AJ. Adsorptive endocytosis mediates the passage of HIV-1 across the blood-brain barrier: evidence for a post-internalization coreceptor. Journal of cell science. 1998;111(Pt 4):533–540. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

