Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck
- PMID: 27718784
- PMCID: PMC5564292
- DOI: 10.1056/NEJMoa1602252
Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck
Abstract
Background: Patients with recurrent or metastatic squamous-cell carcinoma of the head and neck after platinum chemotherapy have a very poor prognosis and limited therapeutic options. Nivolumab, an anti-programmed death 1 (PD-1) monoclonal antibody, was assessed as treatment for this condition.
Methods: In this randomized, open-label, phase 3 trial, we assigned, in a 2:1 ratio, 361 patients with recurrent squamous-cell carcinoma of the head and neck whose disease had progressed within 6 months after platinum-based chemotherapy to receive nivolumab (at a dose of 3 mg per kilogram of body weight) every 2 weeks or standard, single-agent systemic therapy (methotrexate, docetaxel, or cetuximab). The primary end point was overall survival. Additional end points included progression-free survival, rate of objective response, safety, and patient-reported quality of life.
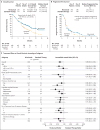
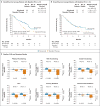
Results: The median overall survival was 7.5 months (95% confidence interval [CI], 5.5 to 9.1) in the nivolumab group versus 5.1 months (95% CI, 4.0 to 6.0) in the group that received standard therapy. Overall survival was significantly longer with nivolumab than with standard therapy (hazard ratio for death, 0.70; 97.73% CI, 0.51 to 0.96; P=0.01), and the estimates of the 1-year survival rate were approximately 19 percentage points higher with nivolumab than with standard therapy (36.0% vs. 16.6%). The median progression-free survival was 2.0 months (95% CI, 1.9 to 2.1) with nivolumab versus 2.3 months (95% CI, 1.9 to 3.1) with standard therapy (hazard ratio for disease progression or death, 0.89; 95% CI, 0.70 to 1.13; P=0.32). The rate of progression-free survival at 6 months was 19.7% with nivolumab versus 9.9% with standard therapy. The response rate was 13.3% in the nivolumab group versus 5.8% in the standard-therapy group. Treatment-related adverse events of grade 3 or 4 occurred in 13.1% of the patients in the nivolumab group versus 35.1% of those in the standard-therapy group. Physical, role, and social functioning was stable in the nivolumab group, whereas it was meaningfully worse in the standard-therapy group.
Conclusions: Among patients with platinum-refractory, recurrent squamous-cell carcinoma of the head and neck, treatment with nivolumab resulted in longer overall survival than treatment with standard, single-agent therapy. (Funded by Bristol-Myers Squibb; CheckMate 141 ClinicalTrials.gov number, NCT02105636 .).
Figures
Comment in
-
Nivolumab for Squamous-Cell Cancer of Head and Neck.N Engl J Med. 2017 Feb 9;376(6):596. doi: 10.1056/NEJMc1615565. N Engl J Med. 2017. PMID: 28177863 No abstract available.
-
Nivolumab for Squamous-Cell Cancer of Head and Neck.N Engl J Med. 2017 Feb 9;376(6):595. doi: 10.1056/NEJMc1615565. N Engl J Med. 2017. PMID: 28181782 No abstract available.
-
Nivolumab for Squamous-Cell Cancer of Head and Neck.N Engl J Med. 2017 Feb 9;376(6):595-6. doi: 10.1056/NEJMc1615565. N Engl J Med. 2017. PMID: 28181785 No abstract available.
-
Immune Checkpoint Inhibition in Cancers that Affect the Head and Neck.Int J Radiat Oncol Biol Phys. 2017 Aug 1;98(5):969-973. doi: 10.1016/j.ijrobp.2017.03.003. Epub 2017 Jul 10. Int J Radiat Oncol Biol Phys. 2017. PMID: 28721906 No abstract available.
-
CheckMate 141 trial: all that glitters is not gold.Expert Opin Biol Ther. 2019 Mar;19(3):169-171. doi: 10.1080/14712598.2019.1570498. Epub 2019 Jan 21. Expert Opin Biol Ther. 2019. PMID: 30652499 No abstract available.
References
-
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86. - PubMed
-
- Pignon JP, le Maître A, Maillard E, Bourhis J. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4–14. - PubMed
-
- Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350:1945–52. - PubMed
-
- Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937–44. - PubMed
-
- Saloura V, Cohen EE, Licitra L, et al. An open-label single-arm, phase II trial of zalutumumab, a human monoclonal anti-EGFR antibody, in patients with platinum-refractory squamous cell carcinoma of the head and neck. Cancer Chemother Pharmacol. 2014;73:1227–39. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
