Distinguishing characteristics of difficult-to-control asthma in inner-city children and adolescents
- PMID: 27720017
- PMCID: PMC5379996
- DOI: 10.1016/j.jaci.2016.06.059
Distinguishing characteristics of difficult-to-control asthma in inner-city children and adolescents
Abstract
Background: Treatment levels required to control asthma vary greatly across a population with asthma. The factors that contribute to variability in treatment requirements of inner-city children have not been fully elucidated.
Objective: We sought to identify the clinical characteristics that distinguish difficult-to-control asthma from easy-to-control asthma.
Methods: Asthmatic children aged 6 to 17 years underwent baseline assessment and bimonthly guideline-based management visits over 1 year. Difficult-to-control and easy-to-control asthma were defined as daily therapy with 500 μg of fluticasone or greater with or without a long-acting β-agonist versus 100 μg or less assigned on at least 4 visits. Forty-four baseline variables were used to compare the 2 groups by using univariate analyses and to identify the most relevant features of difficult-to-control asthma by using a variable selection algorithm. Nonlinear seasonal variation in longitudinal measures (symptoms, pulmonary physiology, and exacerbations) was examined by using generalized additive mixed-effects models.
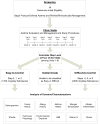
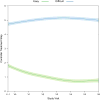
Results: Among 619 recruited participants, 40.9% had difficult-to-control asthma, 37.5% had easy-to-control asthma, and 21.6% fell into neither group. At baseline, FEV1 bronchodilator responsiveness was the most important characteristic distinguishing difficult-to-control asthma from easy-to-control asthma. Markers of rhinitis severity and atopy were among the other major discriminating features. Over time, difficult-to-control asthma was characterized by high exacerbation rates, particularly in spring and fall; greater daytime and nighttime symptoms, especially in fall and winter; and compromised pulmonary physiology despite ongoing high-dose controller therapy.
Conclusions: Despite good adherence, difficult-to-control asthma showed little improvement in symptoms, exacerbations, or pulmonary physiology over the year. In addition to pulmonary physiology measures, rhinitis severity and atopy were associated with high-dose asthma controller therapy requirement.
Keywords: Child; IgE; allergen sensitization; asthma; asthma exacerbations; asthma morbidity; asthma phenotype; asthma severity; inner-city asthma; pulmonary function; rhinitis.
Copyright © 2016 American Academy of Allergy, Asthma & Immunology. All rights reserved.
Figures



Comment in
-
The multimorbid polysensitized phenotype is associated with the severity of allergic diseases.J Allergy Clin Immunol. 2017 Apr;139(4):1407-1408. doi: 10.1016/j.jaci.2016.12.961. Epub 2017 Feb 22. J Allergy Clin Immunol. 2017. PMID: 28237729 No abstract available.
References
-
- Togias A. Rhinitis and asthma: evidence for respiratory system integration. J Allergy Clin Immunol. 2003;111:1171–83. quiz 84. - PubMed
-
- Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120:S94–138. - PubMed
-
- Bousquet J, Khaltaev N, Cruz AA, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen) Allergy. 2008;63(86):8–160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1 TR000150/TR/NCATS NIH HHS/United States
- UL1 TR001105/TR/NCATS NIH HHS/United States
- UL1 TR000075/TR/NCATS NIH HHS/United States
- UL1 RR025780/RR/NCRR NIH HHS/United States
- UL1 TR000154/TR/NCATS NIH HHS/United States
- UM2 AI117870/AI/NIAID NIH HHS/United States
- UL1 TR001082/TR/NCATS NIH HHS/United States
- HHSN272200900052C/AI/NIAID NIH HHS/United States
- HHSN272201000052I/AI/NIAID NIH HHS/United States
- UL1 TR000451/TR/NCATS NIH HHS/United States
- UM1 AI114271/AI/NIAID NIH HHS/United States
- UL1 TR000040/TR/NCATS NIH HHS/United States
- UL1 TR000077/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

