Blast waves from detonated military explosive reduce GluR1 and synaptophysin levels in hippocampal slice cultures
- PMID: 27720798
- PMCID: PMC5767515
- DOI: 10.1016/j.expneurol.2016.10.002
Blast waves from detonated military explosive reduce GluR1 and synaptophysin levels in hippocampal slice cultures
Abstract
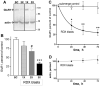
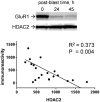
Explosives create shockwaves that cause blast-induced neurotrauma, one of the most common types of traumatic brain injury (TBI) linked to military service. Blast-induced TBIs are often associated with reduced cognitive and behavioral functions due to a variety of factors. To study the direct effects of military explosive blasts on brain tissue, we removed systemic factors by utilizing rat hippocampal slice cultures. The long-term slice cultures were briefly sealed air-tight in serum-free medium, lowered into a 37°C water-filled tank, and small 1.7-gram assemblies of cyclotrimethylene trinitramine (RDX) were detonated 15cm outside the tank, creating a distinct shockwave recorded at the culture plate position. Compared to control mock-treated groups of slices that received equal submerge time, 1-3 blast impacts caused a dose-dependent reduction in the AMPA receptor subunit GluR1. While only a small reduction was found in hippocampal slices exposed to a single RDX blast and harvested 1-2days later, slices that received two consecutive RDX blasts 4min apart exhibited a 26-40% reduction in GluR1, and the receptor subunit was further reduced by 64-72% after three consecutive blasts. Such loss correlated with increased levels of HDAC2, a histone deacetylase implicated in stress-induced reduction of glutamatergic transmission. No evidence of synaptic marker recovery was found at 72h post-blast. The presynaptic marker synaptophysin was found to have similar susceptibility as GluR1 to the multiple explosive detonations. In contrast to the synaptic protein reductions, actin levels were unchanged, spectrin breakdown was not detected, and Fluoro-Jade B staining found no indication of degenerating neurons in slices exposed to three RDX blasts, suggesting that small, sub-lethal explosives are capable of producing selective alterations to synaptic integrity. Together, these results indicate that blast waves from military explosive cause signs of synaptic compromise without producing severe neurodegeneration, perhaps explaining the cognitive and behavioral changes in those blast-induced TBI sufferers that have no detectable neuropathology.
Keywords: Blast-induced injury; GluR1; Military explosive; RDX; Shockwave; Synaptic decline; Synaptophysin; TBI; Traumatic brain injury.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Distinct and dementia-related synaptopathy in the hippocampus after military blast exposures.Brain Pathol. 2021 May;31(3):e12936. doi: 10.1111/bpa.12936. Epub 2021 Feb 24. Brain Pathol. 2021. PMID: 33629462 Free PMC article.
-
Effects on Neurons and Hippocampal Slices by Single and Multiple Primary Blast Pressure Waves From Detonating Spherical Cyclotrimethylenetrinitramine (RDX) Explosive Charges.Mil Med. 2018 Mar 1;183(suppl_1):269-275. doi: 10.1093/milmed/usx158. Mil Med. 2018. PMID: 29635567
-
Primary Blast Injury Depressed Hippocampal Long-Term Potentiation through Disruption of Synaptic Proteins.J Neurotrauma. 2017 Mar 1;34(5):1063-1073. doi: 10.1089/neu.2016.4578. Epub 2016 Oct 13. J Neurotrauma. 2017. PMID: 27573357
-
Injury biomechanics, neuropathology, and simplified physics of explosive blast and impact mild traumatic brain injury.Handb Clin Neurol. 2015;127:89-104. doi: 10.1016/B978-0-444-52892-6.00006-4. Handb Clin Neurol. 2015. PMID: 25702211 Review.
-
Brain injuries from blast.Ann Biomed Eng. 2012 Jan;40(1):185-202. doi: 10.1007/s10439-011-0424-0. Epub 2011 Oct 20. Ann Biomed Eng. 2012. PMID: 22012085 Review.
Cited by
-
Early Synaptic Alterations and Selective Adhesion Signaling in Hippocampal Dendritic Zones Following Organophosphate Exposure.Sci Rep. 2019 Apr 25;9(1):6532. doi: 10.1038/s41598-019-42934-z. Sci Rep. 2019. PMID: 31024077 Free PMC article.
-
Pulsed Microwave Energy Transduction of Acoustic Phonon Related Brain Injury.Front Neurol. 2020 Aug 4;11:753. doi: 10.3389/fneur.2020.00753. eCollection 2020. Front Neurol. 2020. PMID: 32849213 Free PMC article.
-
Neuronal susceptibility to beta-amyloid toxicity and ischemic injury involves histone deacetylase-2 regulation of endophilin-B1.Brain Pathol. 2019 Mar;29(2):164-175. doi: 10.1111/bpa.12647. Epub 2018 Oct 5. Brain Pathol. 2019. PMID: 30028551 Free PMC article.
-
MiRNA expression profiling reveals a potential role of microRNA-148b-3p in cerebral vasospasm in subarachnoid hemorrhage.Sci Rep. 2024 Sep 29;14(1):22539. doi: 10.1038/s41598-024-73579-2. Sci Rep. 2024. PMID: 39341923 Free PMC article.
-
A 6-Step Approach to Gain Higher Quality Results From Organotypic Hippocampal Brain Slices in a Traumatic Brain Injury Model.Basic Clin Neurosci. 2019 Sep-Oct;10(5):485-498. doi: 10.32598/bcn.9.10.235. Epub 2019 Sep 1. Basic Clin Neurosci. 2019. PMID: 32284838 Free PMC article.
References
-
- Arun P, Spadaro J, John J, Gharavi RB, Bentley TB, Nambiar MP. Studies on blast traumatic brain injury using in-vitro model with shock tube. Neurol Rep. 2011;22:379–384. - PubMed
-
- Bahr BA. Long-term hippocampal slices: a model system for investigating synaptic mechanisms and pathologic processes. J Neurosci Res. 1995;42:294–305. - PubMed
-
- Bahr BA, Kessler M, Rivera S, Vanderklish PW, Hall RA, Singh Mutneja M, Gall C, Hoffman KB. Stable maintenance of glutamate receptors and other synaptic components in long-term hippocampal slices. Hippocampus. 1995a;5:425–439. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

