Endogenous DNA Damage Leads to p53-Independent Deficits in Replicative Fitness in Fetal Murine Fancd2-/- Hematopoietic Stem and Progenitor Cells
- PMID: 27720904
- PMCID: PMC5106485
- DOI: 10.1016/j.stemcr.2016.09.005
Endogenous DNA Damage Leads to p53-Independent Deficits in Replicative Fitness in Fetal Murine Fancd2-/- Hematopoietic Stem and Progenitor Cells
Abstract
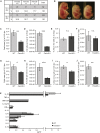
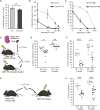
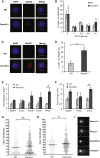
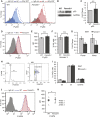
Our mechanistic understanding of Fanconi anemia (FA) pathway function in hematopoietic stem and progenitor cells (HSPCs) owes much to their role in experimentally induced DNA crosslink lesion repair. In bone marrow HSPCs, unresolved stress confers p53-dependent apoptosis and progressive cell attrition. The role of FA proteins during hematopoietic development, in the face of physiological replicative demand, remains elusive. Here, we reveal a fetal HSPC pool in Fancd2-/- mice with compromised clonogenicity and repopulation. Without experimental manipulation, fetal Fancd2-/- HSPCs spontaneously accumulate DNA strand breaks and RAD51 foci, associated with a broad transcriptional DNA-damage response, and constitutive activation of ATM as well as p38 stress kinase. Remarkably, the unresolved stress during rapid HSPC pool expansion does not trigger p53 activation and apoptosis; rather, it constrains proliferation. Collectively our studies point to a role for the FA pathway during hematopoietic development and provide a new model for studying the physiological function of FA proteins.
Keywords: Fanconi anemia; bone marrow failure; development; hematopoiesis; stem cells.
Copyright © 2016 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Impairment of fetal hematopoietic stem cell function in the absence of Fancd2.Exp Hematol. 2017 Apr;48:79-86. doi: 10.1016/j.exphem.2016.11.006. Epub 2016 Dec 1. Exp Hematol. 2017. PMID: 27915139 Free PMC article.
-
Fancd2 and p21 function independently in maintaining the size of hematopoietic stem and progenitor cell pool in mice.Stem Cell Res. 2013 Sep;11(2):687-92. doi: 10.1016/j.scr.2013.04.010. Epub 2013 Apr 30. Stem Cell Res. 2013. PMID: 23721813 Free PMC article.
-
Bone marrow failure in Fanconi anemia is triggered by an exacerbated p53/p21 DNA damage response that impairs hematopoietic stem and progenitor cells.Cell Stem Cell. 2012 Jul 6;11(1):36-49. doi: 10.1016/j.stem.2012.05.013. Epub 2012 Jun 7. Cell Stem Cell. 2012. PMID: 22683204 Free PMC article.
-
Rad18 confers hematopoietic progenitor cell DNA damage tolerance independently of the Fanconi Anemia pathway in vivo.Nucleic Acids Res. 2016 May 19;44(9):4174-88. doi: 10.1093/nar/gkw072. Epub 2016 Feb 15. Nucleic Acids Res. 2016. PMID: 26883629 Free PMC article.
-
Mechanisms of somatic transformation in inherited bone marrow failure syndromes.Hematology Am Soc Hematol Educ Program. 2021 Dec 10;2021(1):390-398. doi: 10.1182/hematology.2021000271. Hematology Am Soc Hematol Educ Program. 2021. PMID: 34889377 Free PMC article. Review.
Cited by
-
An induced pluripotent stem cell model of Fanconi anemia reveals mechanisms of p53-driven progenitor cell differentiation.Blood Adv. 2020 Oct 13;4(19):4679-4692. doi: 10.1182/bloodadvances.2020001593. Blood Adv. 2020. PMID: 33002135 Free PMC article.
-
Studies in an Early Development Window Unveils a Severe HSC Defect in both Murine and Human Fanconi Anemia.Stem Cell Reports. 2018 Nov 13;11(5):1075-1091. doi: 10.1016/j.stemcr.2018.10.001. Epub 2018 Oct 25. Stem Cell Reports. 2018. PMID: 30449320 Free PMC article.
-
Replication stress increases mitochondrial metabolism and mitophagy in FANCD2 deficient fetal liver hematopoietic stem cells.Front Oncol. 2023 Mar 7;13:1108430. doi: 10.3389/fonc.2023.1108430. eCollection 2023. Front Oncol. 2023. PMID: 37007148 Free PMC article.
-
RUNX1 mutations mitigate quiescence to promote transformation of hematopoietic progenitors in Fanconi anemia.Leukemia. 2023 Aug;37(8):1698-1708. doi: 10.1038/s41375-023-01945-6. Epub 2023 Jun 30. Leukemia. 2023. PMID: 37391485 Free PMC article.
-
Deregulated protein homeostasis constrains fetal hematopoietic stem cell pool expansion in Fanconi anemia.Nat Commun. 2024 Feb 29;15(1):1852. doi: 10.1038/s41467-024-46159-1. Nat Commun. 2024. PMID: 38424108 Free PMC article.
References
-
- Alvarez S., Diaz M., Flach J., Rodriguez-Acebes S., Lopez-Contreras A.J., Martinez D., Canamero M., Fernandez-Capetillo O., Isern J., Passegue E. Replication stress caused by low MCM expression limits fetal erythropoiesis and hematopoietic stem cell functionality. Nat. Commun. 2015;6:8548. - PMC - PubMed
-
- Auerbach A.D., Liu Q., Ghosh R., Pollack M.S., Douglas G.W., Broxmeyer H.E. Prenatal identification of potential donors for umbilical cord blood transplantation for Fanconi anemia. Transfusion. 1990;30:682–687. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

