Tumor-associated macrophages drive spheroid formation during early transcoelomic metastasis of ovarian cancer
- PMID: 27721235
- PMCID: PMC5096908
- DOI: 10.1172/JCI87252
Tumor-associated macrophages drive spheroid formation during early transcoelomic metastasis of ovarian cancer
Abstract
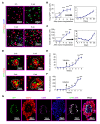
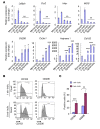
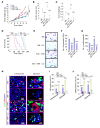
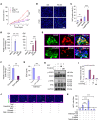
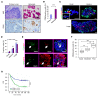
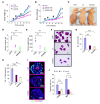
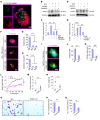
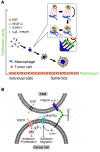
Tumor-associated macrophages (TAMs) can influence ovarian cancer growth, migration, and metastasis, but the detailed mechanisms underlying ovarian cancer metastasis remain unclear. Here, we have shown a strong correlation between TAM-associated spheroids and the clinical pathology of ovarian cancer. Further, we have determined that TAMs promote spheroid formation and tumor growth at early stages of transcoelomic metastasis in an established mouse model for epithelial ovarian cancer. M2 macrophage-like TAMs were localized in the center of spheroids and secreted EGF, which upregulated αMβ2 integrin on TAMs and ICAM-1 on tumor cells to promote association between tumor cells and TAM. Moreover, EGF secreted by TAMs activated EGFR on tumor cells, which in turn upregulated VEGF/VEGFR signaling in surrounding tumor cells to support tumor cell proliferation and migration. Pharmacological blockade of EGFR or antibody neutralization of ICAM-1 in TAMs blunted spheroid formation and ovarian cancer progression in mouse models. These findings suggest that EGF secreted from TAMs plays a critical role in promoting early transcoelomic metastasis of ovarian cancer. As transcoelomic metastasis is also associated with many other cancers, such as pancreatic and colon cancers, our findings uncover a mechanism for TAM-mediated spheroid formation and provide a potential target for the treatment of ovarian cancer and other transcoelomic metastatic cancers.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

