Downregulation of the proapoptotic protein MOAP-1 by the UBR5 ubiquitin ligase and its role in ovarian cancer resistance to cisplatin
- PMID: 27721409
- PMCID: PMC5447866
- DOI: 10.1038/onc.2016.336
Downregulation of the proapoptotic protein MOAP-1 by the UBR5 ubiquitin ligase and its role in ovarian cancer resistance to cisplatin
Abstract
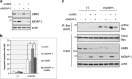
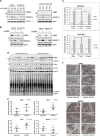
Evasion of apoptosis allows many cancers to resist chemotherapy. Apoptosis is mediated by the serial activation of caspase family proteins. These proteases are often activated upon the release of cytochrome c from the mitochondria, which is promoted by the proapoptotic Bcl-2 family protein, Bax. This function of Bax is enhanced by the MOAP-1 (modulator of apoptosis protein 1) protein in response to DNA damage. Previously, we reported that MOAP-1 is targeted for ubiquitylation and degradation by the APC/CCdh1 ubiquitin ligase. In this study, we identify the HECT (homologous to the E6-AP carboxyl terminus) family E3 ubiquitin ligase, UBR5, as a novel ubiquitin ligase for MOAP-1. We demonstrate that UBR5 interacts physically with MOAP-1, ubiquitylates MOAP-1 in vitro and inhibits MOAP-1 stability in cultured cells. In addition, we show that Dyrk2 kinase, a reported UBR5 interactor, cooperates with UBR5 in mediating MOAP-1 ubiquitylation. Importantly, we found that cisplatin-resistant ovarian cancer cell lines exhibit lower levels of MOAP-1 accumulation than their sensitive counterparts upon cisplatin treatment, consistent with the previously reported role of MOAP-1 in modulating cisplatin-induced apoptosis. Accordingly, UBR5 knockdown increased MOAP-1 expression, enhanced Bax activation and sensitized otherwise resistant cells to cisplatin-induced apoptosis. Furthermore, UBR5 expression was higher in ovarian cancers from cisplatin-resistant patients than from cisplatin-responsive patients. These results show that UBR5 downregulates proapoptotic MOAP-1 and suggest that UBR5 can confer cisplatin resistance in ovarian cancer. Thus UBR5 may be an attractive therapeutic target for ovarian cancer treatment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Comment in
-
MOAP-1, UBR5 and cisplatin resistance in ovarian cancer.Transl Cancer Res. 2017 Feb;6(Suppl 1):S18-S21. doi: 10.21037/tcr.2017.02.01. Transl Cancer Res. 2017. PMID: 29607295 Free PMC article. No abstract available.
References
-
- Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol 2008; 9: 231–241. - PubMed
-
- Tan KO, Tan KM, Chan SL, Yee KS, Bevort M, Ang KC et al. MAP-1, a novel proapoptotic protein containing a BH3-like motif that associates with Bax through its Bcl-2 homology domains. J Biol Chem 2001; 276: 2802–2807. - PubMed
-
- Baksh S, Tommasi S, Fenton S, Yu VC, Martins LM, Pfeifer GP et al. The tumor suppressor RASSF1A and MAP-1 link death receptor signaling to Bax conformational change and cell death. Mol Cell 2005; 18: 637–650. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

