Isocitrate dehydrogenase mutations in myeloid malignancies
- PMID: 27721426
- PMCID: PMC5292675
- DOI: 10.1038/leu.2016.275
Isocitrate dehydrogenase mutations in myeloid malignancies
Abstract
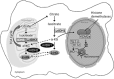
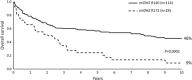
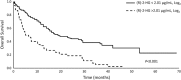
Alterations to genes involved in cellular metabolism and epigenetic regulation are implicated in the pathogenesis of myeloid malignancies. Recurring mutations in isocitrate dehydrogenase (IDH) genes are detected in approximately 20% of adult patients with acute myeloid leukemia (AML) and 5% of adults with myelodysplastic syndromes (MDS). IDH proteins are homodimeric enzymes involved in diverse cellular processes, including adaptation to hypoxia, histone demethylation and DNA modification. The IDH2 protein is localized in the mitochondria and is a critical component of the tricarboxylic acid (also called the 'citric acid' or Krebs) cycle. Both IDH2 and IDH1 (localized in the cytoplasm) proteins catalyze the oxidative decarboxylation of isocitrate to α-ketoglutarate (α-KG). Mutant IDH enzymes have neomorphic activity and catalyze reduction of α-KG to the (R) enantiomer of 2-hydroxyglutarate, which is associated with DNA and histone hypermethylation, altered gene expression and blocked differentiation of hematopoietic progenitor cells. The prognostic significance of mutant IDH (mIDH) is controversial but appears to be influenced by co-mutational status and the specific location of the mutation (IDH1-R132, IDH2-R140, IDH2-R172). Treatments specifically or indirectly targeted to mIDH are currently under clinical investigation; these therapies have been generally well tolerated and, when used as single agents, have shown promise for inducing responses in some mIDH patients when used as first-line treatment or in relapsed or refractory AML or MDS. Use of mIDH inhibitors in combination with drugs with non-overlapping mechanisms of action is especially promising, as such regimens may address the clonal heterogeneity and the multifactorial pathogenic processes involved in mIDH myeloid malignancies. Advances in mutational analysis have made testing more rapid and convenient, and less expensive; such testing should become part of routine diagnostic workup and repeated at relapse to identify patients who may benefit from treatments that target mIDH.
Conflict of interest statement
BCM has received research funding from Celgene and Agios and has received remuneration for Advisory Board participation from Celgene and Agios. ATF is a consultant for and receives clinical research funding from Celgene and declares Advisory Board participation for Agios. CDD has received research funding from Novartis, Celgene, Agios and Abbvie/Genentech and participates in Advisory Boards for Celgene and Agios. DAP has received research funding from Celgene and is a consultant for Celgene, Pfizer, Alexion, Ariad and Karyopharm. SMC has received research funding from Celgene, Agios and Abbvie/Genentech. RS declares Advisory Board participation for Novartis.
Figures
References
-
- Dohner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med 2015; 373: 1136–1152. - PubMed
-
- Albitar M, Manshouri T, Shen Y, Liu D, Beran M, Kantarjian HM et al. Myelodysplastic syndrome is not merely ‘preleukemia'. Blood 2002; 100: 791–798. - PubMed
-
- Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 2009; 114: 937–951. - PubMed
-
- Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer 2011; 11: 85–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

