A prospective, randomized study: Evaluation of the effect of rosuvastatin in patients with chronic obstructive pulmonary disease and pulmonary hypertension
- PMID: 27721534
- PMCID: PMC5051242
- DOI: 10.4103/0253-7613.190721
A prospective, randomized study: Evaluation of the effect of rosuvastatin in patients with chronic obstructive pulmonary disease and pulmonary hypertension
Abstract
Objectives: Statins by their anti-inflammatory and endothelial stabilizing effect can be beneficial in patients with chronic obstructive pulmonary disease (COPD) and pulmonary hypertension (PH). The present study was done to evaluate the effect of rosuvastatin on pulmonary functions and quality of life (QOL) in patients with concomitant COPD and PH.
Materials and methods: It was a prospective, randomized, double-blind, placebo-controlled, study conducted in patients with COPD and PH. A total of sixty patients were assigned to receive either rosuvastatin 10 mg or placebo once a day in addition to their conventional treatment for 12 weeks. Routine blood investigations, pulmonary functions, echocardiogram, exercise capacity, and QOL using a questionnaire were assessed at the baseline and after 12 weeks.
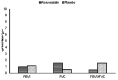
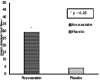
Results: In patients of rosuvastatin group, there was a statistically significant increase in peak expiratory flow rate (PEFR) (P = 0.04) but no significant change in other pulmonary functions: Forced vital capacity (FVC), forced expiratory volume at 1 s (FVC, FEV1, FEV1/FVC), and echocardiogram parameters. There was a significant increase in 6-min walk test (6-min walk distance) (P = 0.03) at the end of 12 weeks. On comparing with placebo, rosuvastatin showed a significant reduction (P = 0.045) in COPD exacerbations while adverse effects did not differ.
Conclusion: Statins have a favorable effect on patients with COPD and PH regarding the improvement in PEFR, COPD exacerbations, and exercise capacity. Such effects can be beneficial in these patients and more so in patients with concomitant coronary artery disease or hyperlipidemia where long-term benefits of statins have been established.
Keywords: Borg's dyspnea score; chronic obstructive pulmonary disease; echocardiogram; hyperlipidemia; pulmonary function tests; quality of life.
Figures
References
-
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for Diagnosis and Management and Prevention of COPD. [Last updated on 2014 Jan 30]. Available from: http://www.goldcopd.org .
-
- Wright JL, Lawson L, Paré PD, Hooper RO, Peretz DI, Nelems JM, et al. The structure and function of the pulmonary vasculature in mild chronic obstructive pulmonary disease. The effect of oxygen and exercise. Am Rev Respir Dis. 1983;128:702–7. - PubMed
-
- Sinden NJ, Stockley RA. Systemic inflammation and comorbidity in COPD: A result of ‘overspill’ of inflammatory mediators from the lungs?. Review of the evidence. Thorax. 2010;65:930–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical