Disentangling a Holobiont - Recent Advances and Perspectives in Nasonia Wasps
- PMID: 27721807
- PMCID: PMC5033955
- DOI: 10.3389/fmicb.2016.01478
Disentangling a Holobiont - Recent Advances and Perspectives in Nasonia Wasps
Abstract
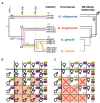
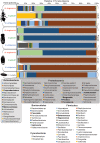
The parasitoid wasp genus Nasonia (Hymenoptera: Chalcidoidea) is a well-established model organism for insect development, evolutionary genetics, speciation, and symbiosis. The host-microbiota assemblage which constitutes the Nasonia holobiont (a host together with all of its associated microbes) consists of viruses, two heritable bacterial symbionts and a bacterial community dominated in abundance by a few taxa in the gut. In the wild, all four Nasonia species are systematically infected with the obligate intracellular bacterium Wolbachia and can additionally be co-infected with Arsenophonus nasoniae. These two reproductive parasites have different transmission modes and host manipulations (cytoplasmic incompatibility vs. male-killing, respectively). Pioneering studies on Wolbachia in Nasonia demonstrated that closely related Nasonia species harbor multiple and mutually incompatible Wolbachia strains, resulting in strong symbiont-mediated reproductive barriers that evolved early in the speciation process. Moreover, research on host-symbiont interactions and speciation has recently broadened from its historical focus on heritable symbionts to the entire microbial community. In this context, each Nasonia species hosts a distinguishable community of gut bacteria that experiences a temporal succession during host development and members of this bacterial community cause strong hybrid lethality during larval development. In this review, we present the Nasonia species complex as a model system to experimentally investigate questions regarding: (i) the impact of different microbes, including (but not limited to) heritable endosymbionts, on the extended phenotype of the holobiont, (ii) the establishment and regulation of a species-specific microbiota, (iii) the role of the microbiota in speciation, and (iv) the resilience and adaptability of the microbiota in wild populations subjected to different environmental pressures. We discuss the potential for easy microbiota manipulations in Nasonia as a promising experimental approach to address these fundamental aspects.
Keywords: Arsenophonus; Nasonia; Wolbachia; host-symbiont interactions; microbiome; symbiosis.
Figures


Similar articles
-
Phylosymbiosis Impacts Adaptive Traits in Nasonia Wasps.mBio. 2019 Jul 16;10(4):e00887-19. doi: 10.1128/mBio.00887-19. mBio. 2019. PMID: 31311878 Free PMC article.
-
Genomes of Gut Bacteria from Nasonia Wasps Shed Light on Phylosymbiosis and Microbe-Assisted Hybrid Breakdown.mSystems. 2021 Apr 6;6(2):e01342-20. doi: 10.1128/mSystems.01342-20. mSystems. 2021. PMID: 33824199 Free PMC article.
-
Nasonia-microbiome associations: a model for evolutionary hologenomics research.Trends Parasitol. 2023 Feb;39(2):101-112. doi: 10.1016/j.pt.2022.11.005. Epub 2022 Dec 7. Trends Parasitol. 2023. PMID: 36496327 Review.
-
The roles of host evolutionary relationships (genus: Nasonia) and development in structuring microbial communities.Evolution. 2012 Feb;66(2):349-62. doi: 10.1111/j.1558-5646.2011.01454.x. Epub 2011 Oct 5. Evolution. 2012. PMID: 22276533
-
The Terrestrial Isopod Microbiome: An All-in-One Toolbox for Animal-Microbe Interactions of Ecological Relevance.Front Microbiol. 2016 Sep 23;7:1472. doi: 10.3389/fmicb.2016.01472. eCollection 2016. Front Microbiol. 2016. PMID: 27721806 Free PMC article. Review.
Cited by
-
Microbiome-mediated plasticity directs host evolution along several distinct time scales.Philos Trans R Soc Lond B Biol Sci. 2020 Sep 28;375(1808):20190589. doi: 10.1098/rstb.2019.0589. Epub 2020 Aug 10. Philos Trans R Soc Lond B Biol Sci. 2020. PMID: 32772662 Free PMC article.
-
Phylosymbiosis Impacts Adaptive Traits in Nasonia Wasps.mBio. 2019 Jul 16;10(4):e00887-19. doi: 10.1128/mBio.00887-19. mBio. 2019. PMID: 31311878 Free PMC article.
-
The mosquito holobiont: fresh insight into mosquito-microbiota interactions.Microbiome. 2018 Mar 20;6(1):49. doi: 10.1186/s40168-018-0435-2. Microbiome. 2018. PMID: 29554951 Free PMC article. Review.
-
Microbiome of the wasp Vespula pensylvanica in native and invasive populations, and associations with Moku virus.PLoS One. 2021 Jul 29;16(7):e0255463. doi: 10.1371/journal.pone.0255463. eCollection 2021. PLoS One. 2021. PMID: 34324610 Free PMC article.
-
Coadaptation between host genome and microbiome under long-term xenobiotic-induced selection.Sci Adv. 2021 May 5;7(19):eabd4473. doi: 10.1126/sciadv.abd4473. Print 2021 May. Sci Adv. 2021. PMID: 33952510 Free PMC article.
References
-
- Balas M. T., Lee M. H., Werren J. H. (1996). Distribution and fitness effects of the son-killer bacterium in Nasonia. Evol. Ecol. 10 593–607. 10.1007/BF01237709 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

