Histomorphological Factors Predicting the Response to Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer
- PMID: 27721875
- PMCID: PMC5053310
- DOI: 10.4048/jbc.2016.19.3.261
Histomorphological Factors Predicting the Response to Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer
Abstract
Purpose: There is no standard targeted therapy for the treatment of triple-negative breast cancer (TNBC). Therefore, its management heavily depends on adjuvant chemotherapy. Using core needle biopsy, this study evaluated the histological factors of TNBC predicting the response to chemotherapy.
Methods: One hundred forty-three TNBC patients who received single-regimen neoadjuvant chemotherapy (NAC) with the combination of doxorubicin, cyclophosphamide, and docetaxel were enrolled. The core needle biopsy specimens acquired before NAC were used to analyze the clinicopathologic variables and overall performance of the predictive model for therapeutic response.
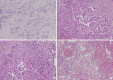
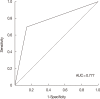
Results: Independent predictors of pathologic complete response after NAC were found to be higher number of tumor infiltrating lymphocytes (p=0.007), absence of clear cytoplasm (p=0.008), low necrosis (p=0.018), and high histologic grade (p=0.039). In the receiver operating characteristics curve analysis, the area under curve for the combination of these four variables was 0.777.
Conclusion: The present study demonstrated that a predictive model using the above four variables can predict therapeutic response to single-regimen NAC with the combination of doxorubicin, cyclophosphamide, and docetaxel in TNBC. Therefore, adding these morphologic variables to clinical and genomic signatures might enhance the ability to predict the therapeutic response to NAC in TNBC.
Keywords: Core needle biopsy; Neoadjuvant therapy; Treatment outcome; Triple-negative breast neoplasms.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Comment in
-
Comment on "Histomorphological Factors Predicting the Response to Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer".J Breast Cancer. 2017 Mar;20(1):114-115. doi: 10.4048/jbc.2017.20.1.114. Epub 2017 Mar 24. J Breast Cancer. 2017. PMID: 28382104 Free PMC article. No abstract available.
References
-
- von Minckwitz G, Raab G, Caputo A, Schütte M, Hilfrich J, Blohmer JU, et al. Doxorubicin with cyclophosphamide followed by docetaxel every 21 days compared with doxorubicin and docetaxel every 14 days as preoperative treatment in operable breast cancer: the GEPARDUO study of the German Breast Group. J Clin Oncol. 2005;23:2676–2685. - PubMed
-
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Clarke M, Coates AS, Darby SC, Davies C, Gelber RD, et al. Adjuvant chemotherapy in oestrogen-receptor-poor breast cancer: patient-level meta-analysis of randomised trials. Lancet. 2008;371:29–40. - PubMed
-
- Torrisi R, Balduzzi A, Ghisini R, Rocca A, Bottiglieri L, Giovanardi F, et al. Tailored preoperative treatment of locally advanced triple negative (hormone receptor negative and HER2 negative) breast cancer with epirubicin, cisplatin, and infusional fluorouracil followed by weekly paclitaxel. Cancer Chemother Pharmacol. 2008;62:667–672. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

