The differential role of cortical protein synthesis in taste memory formation and persistence
- PMID: 27721985
- PMCID: PMC5053367
- DOI: 10.1038/npjscilearn.2016.1
The differential role of cortical protein synthesis in taste memory formation and persistence
Abstract
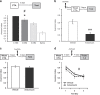
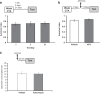
The current dogma suggests that the formation of long-term memory (LTM) is dependent on protein synthesis but persistence of the memory trace is not. However, many of the studies examining the effect of protein synthesis inhibitors (PSIs) on LTM persistence were performed in the hippocampus, which is known to have a time-dependent role in memory storage, rather than the cortex, which is considered to be the main structure to store long-term memories. Here we studied the effect of PSIs on LTM formation and persistence in male Wistar Hola (n ≥ 5) rats by infusing the protein synthesis inhibitor, anisomycin (100 μg, 1 μl), into the gustatory cortex (GC) during LTM formation and persistence in conditioned taste aversion (CTA). We found that local anisomycin infusion to the GC before memory acquisition impaired LTM formation (P = 8.9E - 5), but had no effect on LTM persistence when infused 3 days post acquisition (P = 0.94). However, when we extended the time interval between treatment with anisomycin and testing from 3 days to 14 days, LTM persistence was enhanced (P = 0.01). The enhancement was on the background of stable and non-declining memory, and was not recapitulated by another amnesic agent, APV (10 μg, 1 μl), an N-methyl-d-aspartate receptor antagonist (P = 0.54). In conclusion, CTA LTM remains sensitive to the action of PSIs in the GC even 3 days following memory acquisition. This sensitivity is differentially expressed between the formation and persistence of LTM, suggesting that increased cortical protein synthesis promotes LTM formation, whereas decreased protein synthesis promotes LTM persistence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Dudai, Y. The neurobiology of consolidations, or, how stable is the engram? Annu. Rev. Psychol. 55, 51–86 (2004). - PubMed
-
- Costa-Mattioli, M. , Sonenberg, N. & Richter, J. D. Translational regulatory mechanisms in synaptic plasticity and memory storage. Prog. Mol. Biol. Transl. Sci. 90, 293–311 (2009). - PubMed
-
- Katche, C. , Cammarota, M. & Medina, J. H. Molecular signatures and mechanisms of long-lasting memory consolidation and storage. Neurobiol. Learn. Mem. 106, 40–47 (2013). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

