A multiscale 3D finite element analysis of fluid/solute transport in mechanically loaded bone
- PMID: 27722020
- PMCID: PMC5037578
- DOI: 10.1038/boneres.2016.32
A multiscale 3D finite element analysis of fluid/solute transport in mechanically loaded bone
Abstract
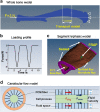
The transport of fluid, nutrients, and signaling molecules in the bone lacunar-canalicular system (LCS) is critical for osteocyte survival and function. We have applied the fluorescence recovery after photobleaching (FRAP) approach to quantify load-induced fluid and solute transport in the LCS in situ, but the measurements were limited to cortical regions 30-50 μm underneath the periosteum due to the constrains of laser penetration. With this work, we aimed to expand our understanding of load-induced fluid and solute transport in both trabecular and cortical bone using a multiscaled image-based finite element analysis (FEA) approach. An intact murine tibia was first re-constructed from microCT images into a three-dimensional (3D) linear elastic FEA model, and the matrix deformations at various locations were calculated under axial loading. A segment of the above 3D model was then imported to the biphasic poroelasticity analysis platform (FEBio) to predict load-induced fluid pressure fields, and interstitial solute/fluid flows through LCS in both cortical and trabecular regions. Further, secondary flow effects such as the shear stress and/or drag force acting on osteocytes, the presumed mechano-sensors in bone, were derived using the previously developed ultrastructural model of Brinkman flow in the canaliculi. The material properties assumed in the FEA models were validated against previously obtained strain and FRAP transport data measured on the cortical cortex. Our results demonstrated the feasibility of this computational approach in estimating the fluid flux in the LCS and the cellular stimulation forces (shear and drag forces) for osteocytes in any cortical and trabecular bone locations, allowing further studies of how the activation of osteocytes correlates with in vivo functional bone formation. The study provides a promising platform to reveal potential cellular mechanisms underlying the anabolic power of exercises and physical activities in treating patients with skeletal deficiencies.
Figures






Similar articles
-
Lactation alters fluid flow and solute transport in maternal skeleton: A multiscale modeling study on the effects of microstructural changes and loading frequency.Bone. 2021 Oct;151:116033. doi: 10.1016/j.bone.2021.116033. Epub 2021 Jun 5. Bone. 2021. PMID: 34102350 Free PMC article.
-
Real-time measurement of solute transport within the lacunar-canalicular system of mechanically loaded bone: direct evidence for load-induced fluid flow.J Bone Miner Res. 2011 Feb;26(2):277-85. doi: 10.1002/jbmr.211. J Bone Miner Res. 2011. PMID: 20715178 Free PMC article.
-
Multiscale interstitial fluid computation modeling of cortical bone to characterize the hydromechanical stimulation of lacunar-canalicular network.Bone. 2025 Apr;193:117386. doi: 10.1016/j.bone.2024.117386. Epub 2024 Dec 31. Bone. 2025. PMID: 39746592
-
Solute Transport in the Bone Lacunar-Canalicular System (LCS).Curr Osteoporos Rep. 2018 Feb;16(1):32-41. doi: 10.1007/s11914-018-0414-3. Curr Osteoporos Rep. 2018. PMID: 29349685 Free PMC article. Review.
-
Would increased interstitial fluid flow through in situ mechanical stimulation enhance bone remodeling?Med Hypotheses. 2010 Aug;75(2):196-8. doi: 10.1016/j.mehy.2010.02.021. Epub 2010 Mar 15. Med Hypotheses. 2010. PMID: 20227836 Review.
Cited by
-
Static Preload Inhibits Loading-Induced Bone Formation.JBMR Plus. 2018 Oct 11;3(5):e10087. doi: 10.1002/jbm4.10087. eCollection 2019 May. JBMR Plus. 2018. PMID: 31131340 Free PMC article.
-
Lactation alters fluid flow and solute transport in maternal skeleton: A multiscale modeling study on the effects of microstructural changes and loading frequency.Bone. 2021 Oct;151:116033. doi: 10.1016/j.bone.2021.116033. Epub 2021 Jun 5. Bone. 2021. PMID: 34102350 Free PMC article.
-
Predicting cortical bone resorption in the mouse tibia under disuse conditions caused by transient muscle paralysis.Sci Rep. 2025 Aug 14;15(1):29884. doi: 10.1038/s41598-025-03588-2. Sci Rep. 2025. PMID: 40813863 Free PMC article.
-
Finite Element Modelling Simulated Meniscus Translocation and Deformation during Locomotion of the Equine Stifle.Animals (Basel). 2019 Jul 31;9(8):502. doi: 10.3390/ani9080502. Animals (Basel). 2019. PMID: 31370196 Free PMC article.
-
Numerical study of interstitial fluid flow behavior in osteons under dynamic loading.BMC Musculoskelet Disord. 2025 Feb 24;26(1):187. doi: 10.1186/s12891-025-08425-1. BMC Musculoskelet Disord. 2025. PMID: 39994737 Free PMC article.
References
-
- Duncan RL , Turner CH . Mechanotransduction and the functional response of bone to mechanical strain. Calcif Tissue Int 1995; 57: 344–358. - PubMed
-
- Klein-Nulend J , Bacabac RG , Mullender MG . Mechanobiology of bone tissue. Pathol Biol (Paris) 2005; 53: 576–580. - PubMed
-
- Lanyon LE , Hampson WG , Goodship AE et al. Bone deformation recorded in vivo from strain gauges attached to the human tibial shaft. Acta Orthop Scand 1975; 46: 256–268. - PubMed
-
- Burr DB , Milgrom C , Fyhrie D et al. In vivo measurement of human tibial strains during vigorous activity. Bone 1996; 18: 405–410. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

