BCL-2 inhibition impairs mitochondrial function and targets oral tongue squamous cell carcinoma
- PMID: 27722045
- PMCID: PMC5031576
- DOI: 10.1186/s40064-016-3310-2
BCL-2 inhibition impairs mitochondrial function and targets oral tongue squamous cell carcinoma
Abstract
Purpose: To understand the role of Bcl-2 overexpression in oral tongue squamous cell carcinoma (OTSCC) patients and investigate the efficacy of targeting Bcl-2 in OTSCC.
Methods: The expression level of Bcl-2 on normal tongue cells and OTSCC cells were measured by real-time PCR and western blotting. The functional roles of Bcl-2 were examined by MTS, flow cytometry and xenograft cancer mouse model. Mechanism studies were performed by analyzing mitochondrial functions in a panel of OTSCC cell lines.
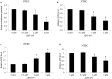
Results: Bcl-2 is up-regulated at mRNA and protein levels in a panel of OTSCC cell lines compared to normal tongue epithelial cells (NTEC). Importantly, overexpression of Bcl-2 confers resistance of OTSCC cells to chemotherapeutic drug cisplatin treatment. Overexpression of Bcl-2 in NTEC significantly increased cell growth. In contrast, inhibition of Bcl-2 by genetic and pharmacological approaches inhibits proliferation and induces apoptosis in OTSCC cells. Mechanistically, Bcl-2 inhibitor ABT-199 impairs mitochondrial functions as shown by the decreased levels of mitochondrial membrane potential, mitochondrial respiration and ATP, and the increased levels of ROS in OTSCC cells. In addition, ABT-199 inhibits proliferation and induces apoptosis and mitochondrial dysfunctions in NTEC cells, but to a less extent than in OTSCC cells. We further show that ABT-199 augments the effects of cisplatin in eliminating OTSCC cells in in vitro tongue cancer cellular system and in vivo tongue cancer xenograft mouse model.
Conclusions: Inhibition of Bcl-2 effectively targets OTSCC cells through inhibiting proliferation and inducing apoptosis. Inhibition of Bcl-2 also augments the inhibitory effects of cisplatin in vitro and in vivo.
Keywords: ABT-199; Bcl-2; Mitochondria; Tongue squamous carcinoma.
Figures






References
-
- Camisasca DR, Honorato J, Bernardo V, da Silva LE, da Fonseca EC, de Faria PA, et al. Expression of Bcl-2 family proteins and associated clinicopathologic factors predict survival outcome in patients with oral squamous cell carcinoma. Oral Oncol. 2009;45(3):225–233. doi: 10.1016/j.oraloncology.2008.05.021. - DOI - PubMed
-
- Domen J, Gandy KL, Weissman IL. Systemic overexpression of BCL-2 in the hematopoietic system protects transgenic mice from the consequences of lethal irradiation. Blood. 1998;91(7):2272–2282. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

