Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy
- PMID: 27722328
- PMCID: PMC5118097
- DOI: 10.1039/c6cs00271d
Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy
Abstract
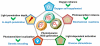
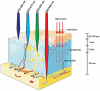
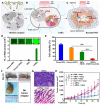
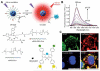
The reactive oxygen species (ROS)-mediated mechanism is the major cause underlying the efficacy of photodynamic therapy (PDT). The PDT procedure is based on the cascade of synergistic effects between light, a photosensitizer (PS) and oxygen, which greatly favors the spatiotemporal control of the treatment. This procedure has also evoked several unresolved challenges at different levels including (i) the limited penetration depth of light, which restricts traditional PDT to superficial tumours; (ii) oxygen reliance does not allow PDT treatment of hypoxic tumours; (iii) light can complicate the phototherapeutic outcomes because of the concurrent heat generation; (iv) specific delivery of PSs to sub-cellular organelles for exerting effective toxicity remains an issue; and (v) side effects from undesirable white-light activation and self-catalysation of traditional PSs. Recent advances in nanotechnology and nanomedicine have provided new opportunities to develop ROS-generating systems through photodynamic or non-photodynamic procedures while tackling the challenges of the current PDT approaches. In this review, we summarize the current status and discuss the possible opportunities for ROS generation for cancer therapy. We hope this review will spur pre-clinical research and clinical practice for ROS-mediated tumour treatments.
Figures















References
-
- Apel K, Hirt H. Annu. Rev. Plant Biol. 2004;55:373–399. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

