Progesterone receptor positivity is a predictor of long-term benefit from adjuvant tamoxifen treatment of estrogen receptor positive breast cancer
- PMID: 27722840
- PMCID: PMC5065613
- DOI: 10.1007/s10549-016-4007-5
Progesterone receptor positivity is a predictor of long-term benefit from adjuvant tamoxifen treatment of estrogen receptor positive breast cancer
Abstract
Purpose: The independent predictive information from progesterone receptor (PgR) positivity for breast cancer treated with tamoxifen has been questioned after an overview by the Early Breast Cancer Trialists' Collaborative Group (EBCTCG). However, the studies in the overview were to a large content performed before modern PgR immunohistochemistry (IHC) was developed. We therefore investigated the predictive value of PgR determined with IHC in estrogen receptor (ER)-positive tumors from patients participating in the Stockholm trial of adjuvant tamoxifen therapy.
Methods: The Stockholm Breast Cancer Study Group conducted a randomized trial during 1976 through 1990 comparing adjuvant tamoxifen versus control. The patients were stratified according to tumor size and lymph node status in high-risk and low-risk groups. In this study, we evaluated 618 patients with ER-positive "low-risk" breast cancer (size ≤ 30 mm, lymph node-negative) for whom PgR was determined by IHC at one pathology laboratory. The median time of follow-up was 21 years.
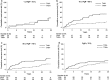
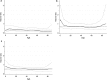
Results: Patients with ER-positive tumors that were also PgR-positive by IHC did benefit from tamoxifen, while we could not show any long-term benefit for those with tumors positive for ER only (recurrence rate ratio 0.43, 95 % CI 0.29-0.62 and 0.87, 95 % CI 0.52-1.46, respectively). We further investigated the influence of different levels of PgR positivity on recurrence risk. The results show that at all receptor levels with ≥10 % stained PgR-positive cells, the patients did benefit from tamoxifen. There was no clear linear trend in benefit with increasing proportion of stained cells.
Conclusions: PgR positivity determined by IHC is a marker indicating long-term benefit from adjuvant tamoxifen in patients with ER-positive tumors.
Keywords: Breast cancer; Estrogen receptor; Progesterone receptor; Tamoxifen.
Conflict of interest statement
The authors declare that they have no conflict of interest. Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the local ethical committee at the Karolinska University Hospital (KI 97-451 with amendment 030201) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent According to the ethical approval informed consent from the patients was not required.
Figures



References
-
- Davies C G, Godwin J, Gray R, Clarke M, Cutter D, Darby S, McGale P, Pan HC, Taylor C, Wang YC, Dowsett M, Ingle J, Peto R, Early Breast Cancer Trialists’Collaborative Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771–784. doi: 10.1016/S0140-6736(11)60993-8. - DOI - PMC - PubMed
-
- Khoshnoud MR, Lofdahl B, Fohlin H, Fornander T, Stal O, Skoog L, Bergh J, Nordenskjold B. Immunohistochemistry compared to cytosol assays for determination of estrogen receptor and prediction of the long-term effect of adjuvant tamoxifen. Breast Cancer Res Treat. 2011;126(2):421–430. doi: 10.1007/s10549-010-1202-7. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

