Baricitinib, Methotrexate, or Combination in Patients With Rheumatoid Arthritis and No or Limited Prior Disease-Modifying Antirheumatic Drug Treatment
- PMID: 27723271
- PMCID: PMC5347954
- DOI: 10.1002/art.39953
Baricitinib, Methotrexate, or Combination in Patients With Rheumatoid Arthritis and No or Limited Prior Disease-Modifying Antirheumatic Drug Treatment
Abstract
Objective: We undertook this phase III study to evaluate baricitinib, an orally administered JAK-1/JAK-2 inhibitor, as monotherapy or combined with methotrexate (MTX) compared to MTX monotherapy in patients with active rheumatoid arthritis (RA) who had received no or minimal conventional synthetic disease-modifying antirheumatic drugs (DMARDs) and who were naive to biologic DMARDs.
Methods: A total of 588 patients were randomized 4:3:4 to receive MTX monotherapy (once weekly), baricitinib monotherapy (4 mg once daily), or the combination of baricitinib and MTX for 52 weeks. The primary end point assessment was a noninferiority comparison of baricitinib monotherapy to MTX monotherapy based on the proportion of patients meeting the American College of Rheumatology 20% improvement criteria (achieving an ACR20 response) at week 24.
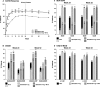
Results: The study met its primary objective. Moreover, baricitinib monotherapy was found to be superior to MTX monotherapy at week 24, with a higher ACR20 response rate (77% versus 62%; P ≤ 0.01). Similar results were observed for combination therapy. Compared to MTX monotherapy, significant improvements in disease activity and physical function were observed for both baricitinib groups as early as week 1. Radiographic progression was reduced in both baricitinib groups compared to MTX monotherapy; the difference was statistically significant for baricitinib plus MTX. The rates of serious adverse events (AEs) were similar across treatment groups, while rates of some treatment-emergent AEs, including infections, were increased with baricitinib plus MTX. Three deaths were reported, all occurring in the MTX monotherapy group. Malignancies, including nonmelanoma skin cancer, were reported in 1 patient receiving MTX monotherapy, 1 receiving baricitinib monotherapy, and 4 receiving baricitinib plus MTX.
Conclusion: Baricitinib alone or in combination with MTX demonstrated superior efficacy with acceptable safety compared to MTX monotherapy as initial therapy for patients with active RA.
Trial registration: ClinicalTrials.gov NCT01711359.
© 2016 The Authors. Arthritis & Rheumatology published by Wiley Periodicals, Inc. on behalf of the American College of Rheumatology.
Figures

References
-
- Colmegna I, Ohata BR, Menard HA. Current understanding of rheumatoid arthritis therapy. Clin Pharmacol Ther 2012;91:607–20. - PubMed
-
- Smolen JS, Steiner G. Therapeutic strategies for rheumatoid arthritis. Nat Rev Drug Discov 2003;2:473–88. - PubMed
-
- Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO III, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. - PubMed
-
- Fatimah N, Salim B, Nasim A, Hussain K, Gul H, Niazi S. Frequency of methotrexate intolerance in rheumatoid arthritis patients using methotrexate intolerance severity score (MISS questionnaire). Clin Rheumatol 2016;35:1341–5. - PubMed
-
- Nikiphorou E, Negoescu A, Fitzpatrick JD, Goudie CT, Badcock A, Östör AJ, et al. Indispensable or intolerable? Methotrexate in patients with rheumatoid and psoriatic arthritis: a retrospective review of discontinuation rates from a large UK cohort. Clin Rheumatol 2014;33:609–14. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

