Successive requirement of Glass and Hazy for photoreceptor specification and maintenance in Drosophila
- PMID: 27723419
- PMCID: PMC5406162
- DOI: 10.1080/19336934.2016.1244591
Successive requirement of Glass and Hazy for photoreceptor specification and maintenance in Drosophila
Abstract
Development of the insect compound eye requires a highly controlled interplay between transcription factors. However, the genetic mechanisms that link early eye field specification to photoreceptor terminal differentiation and fate maintenance remain largely unknown. Here, we decipher the function of 2 transcription factors, Glass and Hazy, which play a central role during photoreceptor development. The regulatory interactions between Glass and Hazy suggest that they function together in a coherent feed-forward loop in all types of Drosophila photoreceptors. While the glass mutant eye lacks the expression of virtually all photoreceptor genes, young hazy mutants correctly express most phototransduction genes. Interestingly, the expression of these genes is drastically reduced in old hazy mutants. This age-dependent loss of the phototransduction cascade correlates with a loss of phototaxis in old hazy mutant flies. We conclude that Glass can either directly or indirectly initiate the expression of most phototransduction proteins in a Hazy-independent manner, and that Hazy is mainly required for the maintenance of functional photoreceptors in adult flies.
Keywords: Drosophila; Glass; Hazy; Pph13; cell fate maintenance; eye development; photoreceptor differentiation; phototransduction.
Figures

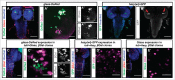

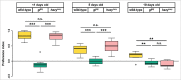
Comment on
- Extra View to: Bernardo-Garcia F. J., Fritsch C., Sprecher S. G. The transcription factor Glass links eye field specification with photoreceptor differentiation in Drosophila. Development 2016; 143:1413-1423.
References
-
- Tsachaki M, Sprecher SG. Genetic and developmental mechanisms underlying the formation of the Drosophila compound eye. Dev Dyn 2012; 241:40-56; PMID:21932322; http://dx.doi.org/10.1002/dvdy.22738 - DOI - PubMed
-
- Treisman JE. Retinal differentiation in Drosophila. Wiley Interdiscip Rev Dev Biol 2013; 2:545-57; PMID:24014422; http://dx.doi.org/10.1002/wdev.100 - DOI - PMC - PubMed
-
- Potier D, Davie K, Hulselmans G, Naval Sanchez M, Haagen L, Huynh-Thu VA, Koldere D, Celik A, Geurts P, Christiaens V, et al.. Mapping gene regulatory networks in Drosophila eye development by large-scale transcriptome perturbations and motif inference. Cell Rep 2014; 9:2290-303; PMID:25533349; http://dx.doi.org/10.1016/j.celrep.2014.11.038 - DOI - PubMed
-
- Halder G, Callaerts P, Gehring WJ. Induction of ectopic eyes by targeted expression of the eyeless gene in Drosophila. Science 1995; 267:1788-92; PMID:7892602; http://dx.doi.org/10.1126/science.7892602 - DOI - PubMed
-
- Pignoni F, Hu B, Zavitz KH, Xiao J, Garrity PA, Zipursky SL. The eye-specification proteins So and Eya form a complex and regulate multiple steps in Drosophila eye development. Cell 1997; 91:881-91; PMID:9428512; http://dx.doi.org/10.1016/S0092-8674(00)80480-8 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
