Microenvironment-dependent growth of preneoplastic and malignant plasma cells in humanized mice
- PMID: 27723723
- PMCID: PMC5101153
- DOI: 10.1038/nm.4202
Microenvironment-dependent growth of preneoplastic and malignant plasma cells in humanized mice
Abstract
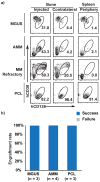
Most human cancers, including myeloma, are preceded by a precursor state. There is an unmet need for in vivo models to study the interaction of human preneoplastic cells in the bone marrow microenvironment with non-malignant cells. Here, we genetically humanized mice to permit the growth of primary human preneoplastic and malignant plasma cells together with non-malignant cells in vivo. Growth was largely restricted to the bone marrow, mirroring the pattern in patients with myeloma. Xenografts captured the genomic complexity of parental tumors and revealed additional somatic changes. Moreover, xenografts from patients with preneoplastic gammopathy showed progressive growth, suggesting that the clinical stability of these lesions may in part be due to growth controls extrinsic to tumor cells. These data demonstrate a new approach to investigate the entire spectrum of human plasma cell neoplasia and illustrate the utility of humanized models for understanding the functional diversity of human tumors.
Figures





References
-
- Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2010;364:1046–1060. - PubMed
-
- Schuler J, Ewerth D, Waldschmidt J, Wasch R, Engelhardt M. Preclinical models of multiple myeloma: a critical appraisal. Expert Opin Biol Ther. 2013;13(Suppl 1):S111–123. - PubMed
-
- Yaccoby S, Barlogie B, Epstein J. Primary myeloma cells growing in SCID-hu mice: a model for studying the biology and treatment of myeloma and its manifestations. Blood. 1998;92:2908–2913. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

