Single-platelet nanomechanics measured by high-throughput cytometry
- PMID: 27723740
- PMCID: PMC5266633
- DOI: 10.1038/nmat4772
Single-platelet nanomechanics measured by high-throughput cytometry
Abstract
Haemostasis occurs at sites of vascular injury, where flowing blood forms a clot, a dynamic and heterogeneous fibrin-based biomaterial. Paramount in the clot's capability to stem haemorrhage are its changing mechanical properties, the major drivers of which are the contractile forces exerted by platelets against the fibrin scaffold. However, how platelets transduce microenvironmental cues to mediate contraction and alter clot mechanics is unknown. This is clinically relevant, as overly softened and stiffened clots are associated with bleeding and thrombotic disorders. Here, we report a high-throughput hydrogel-based platelet-contraction cytometer that quantifies single-platelet contraction forces in different clot microenvironments. We also show that platelets, via the Rho/ROCK pathway, synergistically couple mechanical and biochemical inputs to mediate contraction. Moreover, highly contractile platelet subpopulations present in healthy controls are conspicuously absent in a subset of patients with undiagnosed bleeding disorders, and therefore may function as a clinical diagnostic biophysical biomarker.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
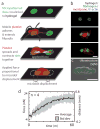

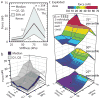
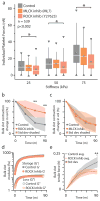
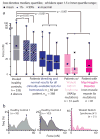
References
-
- Jen CJ, McIntire LV. The structural properties and contractile force of a clot. Cell Motil. 1982;2:445–55. - PubMed
-
- Collet JP, et al. Altered fibrin architecture is associated with hypofibrinolysis and premature coronary atherothrombosis. Arterioscler Thromb Vasc Biol. 2006;26:2567–73. - PubMed
-
- Carr ME. Development of platelet contractile force as a research and clinical measure of platelet function. Cell Biochem Biophys. 2003;38:55–78. - PubMed
-
- Cohen I, De Vries A. Platelet contractile regulation in an isometric system. Nature. 1973;246:36–7. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

