Competing charge transfer pathways at the photosystem II-electrode interface
- PMID: 27723748
- PMCID: PMC5113757
- DOI: 10.1038/nchembio.2192
Competing charge transfer pathways at the photosystem II-electrode interface
Abstract
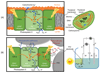
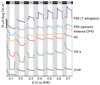
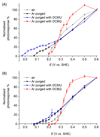
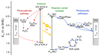
The integration of the water-oxidation enzyme photosystem II (PSII) into electrodes allows the electrons extracted from water oxidation to be harnessed for enzyme characterization and to drive novel endergonic reactions. However, PSII continues to underperform in integrated photoelectrochemical systems despite extensive optimization efforts. Here we carried out protein-film photoelectrochemistry using spinach and Thermosynechococcus elongatus PSII, and we identified a competing charge transfer pathway at the enzyme-electrode interface that short-circuits the known water-oxidation pathway. This undesirable pathway occurs as a result of photo-induced O2 reduction occurring at the chlorophyll pigments and is promoted by the embedment of PSII in an electron-conducting fullerene matrix, a common strategy for enzyme immobilization. Anaerobicity helps to recover the PSII photoresponse and unmasks the onset potentials relating to the QA/QB charge transfer process. These findings impart a fuller understanding of the charge transfer pathways within PSII and at photosystem-electrode interfaces, which will lead to more rational design of pigment-containing photoelectrodes in general.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

Comment in
-
Photosynthesis: Short circuit at the chlorophyll.Nat Chem Biol. 2016 Nov 15;12(12):990-991. doi: 10.1038/nchembio.2240. Nat Chem Biol. 2016. PMID: 27846203 No abstract available.
References
-
- Suga M, et al. Native structure of photosystem II at 1.95 Å resolution viewed by femtosecond X-ray pulses. Nature. 2015;517:99–103. - PubMed
-
- Nath K, et al. Towards a critical understanding of the photosystem II repair mechanism and its regulation during stress conditions. FEBS Lett. 2013;587:3372–3381. - PubMed
-
- Kruse O, Rupprecht J, Mussgnug JH, Dismukes GC, Hankamer B. Photosynthesis: a blueprint for solar energy capture and biohydrogen production technologies. Photochem Photobiol Sci. 2005;4:957–970. - PubMed
-
- Kato M, Zhang JZ, Paul N, Reisner E. Protein film photoelectrochemistry of the water oxidation enzyme photosystem II. Chem Soc Rev. 2014;43:6485–6497. - PubMed
-
- Badura A, Kothe T, Schuhmann W, Rogner M. Wiring photosynthetic enzymes to electrodes. Energy Environ Sci. 2011;4:3263–3274.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

