Effects of probiotics (Vivomixx®) in obese pregnant women and their newborn: study protocol for a randomized controlled trial
- PMID: 27724923
- PMCID: PMC5057415
- DOI: 10.1186/s13063-016-1617-5
Effects of probiotics (Vivomixx®) in obese pregnant women and their newborn: study protocol for a randomized controlled trial
Abstract
Background: Maternal obesity is associated with increased risks of adverse pregnancy-related complications and outcomes for both mothers and infants. Overweight and obese women have an increased risk of pregnancy-induced hypertension, preeclampsia and gestational diabetes mellitus (GDM). Infant Body Mass index (BMI) and the risk of obesity in adulthood are related to maternal gestational weight gain (GWG). Preventive lifestyle and dietary interventions are time-consuming and do not always reduce GWG or the risk of maternal pregnancy complications. Recent research has indicated that the gut microbiota may play a significant role in the development of obesity. Some studies have indicated that the daily consumption of probiotics may reduce the risk of preeclampsia, maintain serum insulin levels and reduce the frequency of GDM in pregnant women. The aims of this study are to investigate whether daily probiotic supplements in obese women during pregnancy can limit gestational weight gain, improve glucose homeostasis and thereby improve maternal, fetal and infant health outcomes.
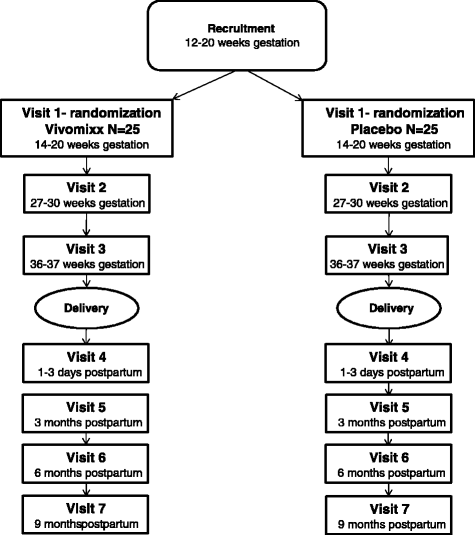
Methods: A pilot study including 50 obese pregnant nulliparous women with a prepregnancy BMI of between 30 and 35 kg/m2 will be randomized to receive daily probiotics (four capsules of Vivomixx®; total of 450 billion CFU/day, including eight probiotic bacterial strains) or placebo from gestational age 14-20 weeks until delivery. The infants will be followed until 9 months of age. The women will be monitored by weight, blood, fecal, vaginal and urine samples, diet questionnaires and hospital record review. Primary outcomes are: maternal weight gain, glycated hemoglobin (HbA1c) level and changes in glucose concentration measured during an oral glucose tolerance test. Secondary outcomes are: microbiota and inflammatory markers in mother and child, pregnancy complications, pregnancy outcomes, physical activity and the body composition of the neonate.
Discussion: We expect to find alterations in the metabolic profiles, microbiota and possibly pregnancy outcomes. From a clinical point of view the effects of Vivomixx® could control weight gain and reduce complications during pregnancy by inducing changes in the gut microbiota. Furthermore, this intervention during pregnancy could influence the infant's microbiota, which could have important implications for infant development and health.
Trial registration: ClincalTrials.gov Identifier: NCT02508844 , registered on 11 May 2015.
Keywords: Gestational diabetes mellitus; Microbiota; Obesity; Pregnancy; Probiotics; Study protocol; Vivomixx®.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical