Diabetes regulates fructose absorption through thioredoxin-interacting protein
- PMID: 27725089
- PMCID: PMC5059142
- DOI: 10.7554/eLife.18313
Diabetes regulates fructose absorption through thioredoxin-interacting protein
Abstract
Metabolic studies suggest that the absorptive capacity of the small intestine for fructose is limited, though the molecular mechanisms controlling this process remain unknown. Here we demonstrate that thioredoxin-interacting protein (Txnip), which regulates glucose homeostasis in mammals, binds to fructose transporters and promotes fructose absorption by the small intestine. Deletion of Txnip in mice reduced fructose transport into the peripheral bloodstream and liver, as well as the severity of adverse metabolic outcomes resulting from long-term fructose consumption. We also demonstrate that fructose consumption induces expression of Txnip in the small intestine. Diabetic mice had increased expression of Txnip in the small intestine as well as enhanced fructose uptake and transport into the hepatic portal circulation. The deletion of Txnip in mice abolished the diabetes-induced increase in fructose absorption. Our results indicate that Txnip is a critical regulator of fructose metabolism and suggest that a diabetic state can promote fructose uptake.
Keywords: GLUT2; GLUT5; Txnip; biochemistry; cell biology; diabetes; fructose; metabolism; mouse.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures


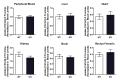


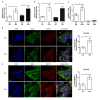
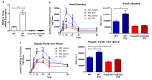
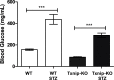
Comment in
-
Keeping tabs on fructose.Elife. 2016 Oct 11;5:e21263. doi: 10.7554/eLife.21263. Elife. 2016. PMID: 27725088 Free PMC article.
-
Metabolism: Diabetes mellitus promotes hepatic fructose uptake.Nat Rev Endocrinol. 2016 Dec;12(12):688. doi: 10.1038/nrendo.2016.181. Epub 2016 Nov 3. Nat Rev Endocrinol. 2016. PMID: 27808273 No abstract available.
References
-
- Abdelmalek MF, Suzuki A, Guy C, Unalp-Arida A, Colvin R, Johnson RJ, Diehl AM, Nonalcoholic Steatohepatitis Clinical Research Network Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51:1961–1971. doi: 10.1002/hep.23535. - DOI - PMC - PubMed
-
- Burant CF, Takeda J, Brot-Laroche E, Bell GI, Davidson NO. Fructose transporter in human spermatozoa and small intestine is GLUT5. Journal of Biological Chemistry. 1992;267:14523–14526. - PubMed
-
- Cha-Molstad H, Saxena G, Chen J, Shalev A. Glucose-stimulated expression of Txnip is mediated by carbohydrate response element-binding protein, p300, and histone H4 acetylation in pancreatic beta cells. Journal of Biological Chemistry. 2009;284:16898–16905. doi: 10.1074/jbc.M109.010504. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

