Production of α1,3-galactosyltransferase and cytidine monophosphate-N-acetylneuraminic acid hydroxylase gene double-deficient pigs by CRISPR/Cas9 and handmade cloning
- PMID: 27725344
- PMCID: PMC5320426
- DOI: 10.1262/jrd.2016-079
Production of α1,3-galactosyltransferase and cytidine monophosphate-N-acetylneuraminic acid hydroxylase gene double-deficient pigs by CRISPR/Cas9 and handmade cloning
Abstract
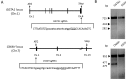
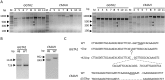
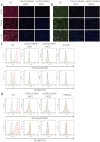
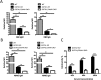
Gene-knockout pigs hold great promise as a solution to the shortage of organs from donor animals for xenotransplantation. Several groups have generated gene-knockout pigs via clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated 9 (Cas9) and somatic cell nuclear transfer (SCNT). Herein, we adopted a simple and micromanipulator-free method, handmade cloning (HMC) instead of SCNT, to generate double gene-knockout pigs. First, we applied the CRISPR/Cas9 system to target α1,3-galactosyltransferase (GGTA1) and cytidine monophosphate-N-acetylneuraminic acid hydroxylase (CMAH) genes simultaneously in porcine fetal fibroblast cells (PFFs), which were derived from wild-type Chinese domestic miniature Wuzhishan pigs. Cell colonies were obtained by screening and were identified by Surveyor assay and sequencing. Next, we chose the GGTA1/CMAH double-knockout (DKO) cells for HMC to produce piglets. As a result, we obtained 11 live bi-allelic GGTA1/CMAH DKO piglets with the identical phenotype. Compared to cells from GGTA1-knockout pigs, human antibody binding and antibody-mediated complement-dependent cytotoxicity were significantly reduced in cells from GGTA1/CMAH DKO pigs, which demonstrated that our pigs would exhibit reduced humoral rejection in xenotransplantation. These data suggested that the combination of CRISPR/Cas9 and HMC technology provided an efficient and new strategy for producing pigs with multiple genetic modifications.
Figures

Similar articles
-
Inclusion of homologous DNA in nuclease-mediated gene targeting facilitates a higher incidence of bi-allelically modified cells.Xenotransplantation. 2015 Sep-Oct;22(5):379-90. doi: 10.1111/xen.12194. Xenotransplantation. 2015. PMID: 26381494 Free PMC article.
-
Efficient production of biallelic GGTA1 knockout pigs by cytoplasmic microinjection of CRISPR/Cas9 into zygotes.Xenotransplantation. 2016 Sep;23(5):338-46. doi: 10.1111/xen.12258. Epub 2016 Sep 9. Xenotransplantation. 2016. PMID: 27610605
-
Generation of α1,3-galactosyltransferase and cytidine monophospho-N-acetylneuraminic acid hydroxylase gene double-knockout pigs.J Reprod Dev. 2015;61(5):449-57. doi: 10.1262/jrd.2015-058. Epub 2015 Jul 26. J Reprod Dev. 2015. PMID: 26227017 Free PMC article.
-
Modifying the sugar icing on the transplantation cake.Glycobiology. 2016 Jun;26(6):571-81. doi: 10.1093/glycob/cww028. Epub 2016 Mar 1. Glycobiology. 2016. PMID: 26935763 Free PMC article. Review.
-
Clustered Regularly Interspaced Short Palindromic Repeats/Cas9 Gene Editing Technique in Xenotransplantation.Front Immunol. 2018 Sep 5;9:1711. doi: 10.3389/fimmu.2018.01711. eCollection 2018. Front Immunol. 2018. PMID: 30233563 Free PMC article. Review.
Cited by
-
Advances in pig models of human diseases.Animal Model Exp Med. 2022 Apr;5(2):141-152. doi: 10.1002/ame2.12223. Epub 2022 Mar 27. Animal Model Exp Med. 2022. PMID: 35343091 Free PMC article. Review.
-
Advances in CRISPR-Based Functional Genomics and Nucleic Acid Detection in Pigs.Front Genet. 2022 May 31;13:891098. doi: 10.3389/fgene.2022.891098. eCollection 2022. Front Genet. 2022. PMID: 35711930 Free PMC article. No abstract available.
-
Xenotransplantation: past, present, and future.Curr Opin Organ Transplant. 2017 Dec;22(6):513-521. doi: 10.1097/MOT.0000000000000463. Curr Opin Organ Transplant. 2017. PMID: 28901971 Free PMC article. Review.
-
Efficient generation of GGTA1-deficient pigs by electroporation of the CRISPR/Cas9 system into in vitro-fertilized zygotes.BMC Biotechnol. 2020 Aug 18;20(1):40. doi: 10.1186/s12896-020-00638-7. BMC Biotechnol. 2020. PMID: 32811500 Free PMC article.
-
Reducing porcine corneal graft rejection, with an emphasis on porcine endogenous retrovirus transmission safety: a review.Int J Ophthalmol. 2019 Feb 18;12(2):324-332. doi: 10.18240/ijo.2019.02.21. eCollection 2019. Int J Ophthalmol. 2019. PMID: 30809491 Free PMC article. Review.
References
-
- Butler JR, Ladowski JM, Martens GR, Tector M, Tector AJ. Recent advances in genome editing and creation of genetically modified pigs. Int J Surg 2015; 23(Pt B): 217–222. - PubMed
-
- DeMayo FJ, Spencer TE. Editors-in-Chief BoR. CRISPR bacon: a sizzling technique to generate genetically engineered pigs. Biol Reprod2014; 91:79. - PubMed
-
- Lutz AJ, Li P, Estrada JL, Sidner RA, Chihara RK, Downey SM, Burlak C, Wang ZY, Reyes LM, Ivary B, Yin F, Blankenship RL, Paris LL, Tector AJ. Double knockout pigs deficient in N-glycolylneuraminic acid and galactose α-1,3-galactose reduce the humoral barrier to xenotransplantation. Xenotransplantation 2013; 20: 27–35. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

