Identification of HRAS as cancer-promoting gene in gastric carcinoma cell aggressiveness
- PMID: 27725900
- PMCID: PMC5043104
Identification of HRAS as cancer-promoting gene in gastric carcinoma cell aggressiveness
Abstract
Gastric carcinoma is one of the most lethal malignancies of cancers and its prognosis remains dismal due to the paucity of effective therapeutic targets. Herein, we showed that HRAS is markedly up-regulated in gastric carcinoma. Prognostic analysis indicated that HRAS expression might be a prognostic indicator for the survival of patients with gastric carcinoma. Ectopic expression of HRAS in gastric carcinoma cells accelerated proliferation, migration, invasion, angiogenesis, and clone formation ability of gastric carcinoma cells in vitro. Furthermore, HRAS over-expressing significantly promoted the tumorigenicity of gastric carcinoma cells in vivo whereas silencing endogenous HRAS caused opposite outcomes. Moreover, we demonstrated that HRAS enhanced gastric carcinoma aggressiveness by activating VEGFA/PI3K/AKT pathway and Raf-1 signaling. Together, our results provide new evidence that HRAS overexpression promotes the progression of gastric carcinoma and might represent a novel therapeutic target for its treatment.
Keywords: HRAS; angiogenesis; gastric carcinoma; growth.
Figures


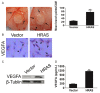
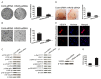


References
-
- Tang J, Wang X, Wang T, Chen F, Zhou J. In vivo pharmacokinetics, biodistribution and antitumor effect of amphiphilic poly(L-amino acids) micelles loaded with a novel all-trans retinoic acid derivative. Eur J Pharm Sci. 2014;51:157–164. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
