Interventions for idiopathic steroid-resistant nephrotic syndrome in children
- PMID: 27726125
- PMCID: PMC6457874
- DOI: 10.1002/14651858.CD003594.pub5
Interventions for idiopathic steroid-resistant nephrotic syndrome in children
Update in
-
Interventions for idiopathic steroid-resistant nephrotic syndrome in children.Cochrane Database Syst Rev. 2019 Nov 21;2019(11):CD003594. doi: 10.1002/14651858.CD003594.pub6. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2025 May 8;5:CD003594. doi: 10.1002/14651858.CD003594.pub7. PMID: 31749142 Free PMC article. Updated.
Abstract
Background: The majority of children who present with their first episode of nephrotic syndrome achieve remission with corticosteroid therapy. Children who fail to respond may be treated with immunosuppressive agents including calcineurin inhibitors (cyclosporin or tacrolimus) and with non-immunosuppressive agents such as angiotensin-converting enzyme inhibitors (ACEi). Optimal combinations of these agents with the least toxicity remain to be determined. This is an update of a review first published in 2004 and updated in 2006 and 2010.
Objectives: To evaluate the benefits and harms of different interventions used in children with idiopathic nephrotic syndrome, who do not achieve remission following four weeks or more of daily corticosteroid therapy.
Search methods: We searched Cochrane Kidney and Transplant's Specialised Register (up to 2 March 2016) through contact with the Information Specialist using search terms relevant to this review.
Selection criteria: RCTs and quasi-RCTs were included if they compared different immunosuppressive agents or non-immunosuppressive agents with placebo, prednisone or other agent given orally or parenterally in children aged three months to 18 years with SRNS.
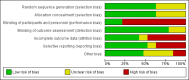
Data collection and analysis: Two authors independently searched the literature, determined study eligibility, assessed risk of bias and extracted data. For dichotomous outcomes, results were expressed as risk ratios (RR) and 95% confidence intervals (CI). Data were pooled using the random effects model.
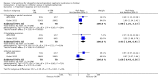
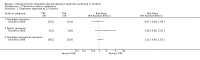
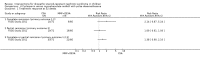
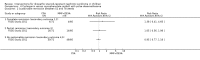
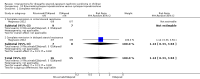
Main results: Nineteen RCTs (820 children enrolled; 773 evaluated) were included. Most studies were small. Eleven studies were at low risk of bias for allocation concealment and only four studies were at low risk of performance bias. Fifteen, eight and 10 studies were at low risk of detection bias, attrition bias and reporting bias respectively. Cyclosporin when compared with placebo or no treatment significantly increased the number of children who achieved complete remission. However this was based on only eight children who achieved remission with cyclosporin compared with no children who achieved remission with placebo/no treatment in three small studies (49 children: RR 7.66, 95% CI 1.06 to 55.34). Calcineurin inhibitors significantly increased the number with complete or partial remission compared with IV cyclophosphamide (2 studies, 156 children: RR 1.98, 95% CI 1.25 to 3.13; I2 = 20%). There was no significant differences in the number who achieved complete remission between tacrolimus versus cyclosporin (1 study, 41 children: RR 0.86, 95% CI 0.44 to 1.66), cyclosporin versus mycophenolate mofetil plus dexamethasone (1 study, 138 children: RR 2.14, 95% CI 0.87 to 5.24), oral cyclophosphamide with prednisone versus prednisone alone (2 studies, 91 children: RR 1.06, 95% CI 0.61 to 1.87), IV versus oral cyclophosphamide (1 study, 11 children: RR 3.13, 95% CI 0.81 to 12.06), IV cyclophosphamide versus oral cyclophosphamide plus IV dexamethasone (1 study, 49 children: RR 1.13, 95% CI 0.65 to 1.96), and azathioprine with prednisone versus prednisone alone (1 study, 31 children: RR 0.94, 95% CI 0.15 to 5.84). One study found no significant differences between three agents (cyclophosphamide, mycophenolate mofetil, leflunomide) used in combination with tacrolimus and prednisone. One study found no significant difference in the percentage reduction in proteinuria (31 children: -12; 95% CI -73 to 110) between rituximab with cyclosporin/prednisolone and cyclosporin/prednisolone alone. Two studies reported ACEi significantly reduced proteinuria.
Authors' conclusions: To date RCTs have demonstrated that calcineurin inhibitors increase the likelihood of complete or partial remission compared with placebo/no treatment or cyclophosphamide. For other regimens assessed, it remains uncertain whether the interventions alter outcomes because the certainty of the evidence is low. Further adequately powered, well designed RCTs are needed to evaluate other regimens for children with idiopathic SRNS. Since SRNS represents a spectrum of diseases, future studies should enrol children from better defined groups of patients with SRNS.
Conflict of interest statement
Elisabeth Hodson: none known
Sophia Wong: none known
Narelle Willis: none known
Jonathan Craig: none known
Figures










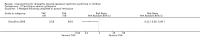









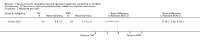



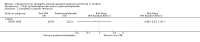
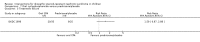

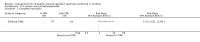








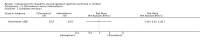






Update of
-
Interventions for idiopathic steroid-resistant nephrotic syndrome in children.Cochrane Database Syst Rev. 2010 Nov 10;(11):CD003594. doi: 10.1002/14651858.CD003594.pub4. Cochrane Database Syst Rev. 2010. Update in: Cochrane Database Syst Rev. 2016 Oct 11;10:CD003594. doi: 10.1002/14651858.CD003594.pub5. PMID: 21069676 Updated.
References
References to studies included in this review
-
- Plank C, Kalb V, Hinkes B, Hildebrandt F, Gefeller O, Rascher W, et al. Cyclosporin A is superior to cyclophosphamide in children with steroid‐resistant nephrotic syndrome‐a randomized controlled multicentre trial by the Arbeitgemeinschaft fur Padiatrische Nephrologie. Pediatric Nephrology 2008;23(9):1483‐93. [MEDLINE: ] - PMC - PubMed
-
- Bagga A, Mudigoudar BD, Hari P, Vasudev V. Enalapril dosage in steroid‐resistant nephrotic syndrome. Pediatric Nephrology 2004;19(1):45‐50. [MEDLINE: ] - PubMed
- Bagga A, Mudigoudar BD, Vasudev V, Hari P. Low (LD) versus high dose (HD) enalapril therapy in steroid resistant nephrotic syndrome (SRNS). Seventh Asian Congress of Pediatric Nephrology; 2000 Nov 4‐6; Singapore. 2000. [CENTRAL: CN‐00583291]
-
- Chongviriyaphan N, Tapaneya‐Olarn C, Suthutvoravut U, Karnchanachumpol S, Chantraruksa V. Effects of tuna fish oil on hyperlipidemia and proteinuria in childhood nephrotic syndrome. Journal of the Medical Association of Thailand 1999;82 Suppl(1):122‐8. [MEDLINE: ] - PubMed
-
- Choudhry A, Bagga A, Menon S, Hari P. Randomized controlled trial (RCT) on efficacy and safety of cyclosporin (CyA) vs tacrolimus (Tac) in steroid resistant nephrotic syndrome (SRNS) [CRG030600042] [abstract no: 182 (OP)]. Pediatric Nephrology 2007;22(9):1480. [CENTRAL: CN‐00653722]
- Choudhry S, Bagga A, Hari P, Sharma S, Kalaivani M, Dinda A. Efficacy and safety of tacrolimus versus cyclosporine in children with steroid‐resistant nephrotic syndrome: a randomized controlled trial. American Journal of Kidney Diseases 2009;53(5):760‐9. [MEDLINE: ] - PubMed
-
- Elhence R, Gulati S, Kher V, Gupta A, Sharma RK. Intravenous pulse cyclophosphamide ‐ a new regime for steroid‐resistant minimal change nephrotic syndrome. Pediatric Nephrology 1994;8(1):1‐3. [MEDLINE: ] - PubMed
- Gupta A, Elhence R, Kher V, Gulati S, Sharma RK. IV cyclophosphamide ‐ a new regimen for steroid nonresponsive minimal change disease [abstract]. 12th International Congress of Nephrology; 1993 Jun 13‐18; Jerusalem, Israel. 1993:84.
References to studies excluded from this review
-
- Arora A, Ahlawat RS, Arora S, Arora N, Mandel AK. Randomised controlled study of enalapril in steroid resistant nephrotic syndrome. Indian Journal of Nephrology 2002;12(3):107‐12. [CENTRAL: CN‐00460300]
-
- Bhaumik SK, Barman SC. Comparison of pulse methyl prednisolone vs cyclosporin based therapy in steroid resistant focal segmental glomerulosclerosis [abstract]. Indian Journal of Nephrology 2002;12(4):190. [CENTRAL: CN‐00460392]
-
- Buyukcelik M, Anarat A, Bayazit AK, Noyan A, Ozel A, Anarat R, et al. The effects of gemfibrozil on hyperlipidemia in children with persistent nephrotic syndrome. Turkish Journal of Pediatrics 2002;44(1):40‐4. [MEDLINE: ] - PubMed
-
- Hiraoka M, Sudo M, West Japanese Cooperative Study of Kidney Disease in Children. Low versus standard dosage of prednisolone for initial treatment of idiopathic nephrotic syndrome in children [abstract no: A0445]. Journal of the American Society of Nephrology 1996;7(9):1335.
- Hiraoka M, Tsukahara H, Haruki S, Hayashi S, Takeda N, Miyagawa K, et al. Older boys benefit from higher initial prednisolone therapy for nephrotic syndrome. The West Japan Cooperative Study of Kidney Disease in Children. Kidney International 2000;58(3):1247‐52. [MEDLINE: ] - PubMed
- Hiraoka M, Tsukahara H, Haruki S, Hayashi S, Takeda N, Miyagawa K, et al. Older boys benefit from intensive initial prednisolone therapy for nephrotic syndrome [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):103A. [CENTRAL: CN‐00550605]
References to ongoing studies
-
- Trachtman H. Efficacy and safety of RE‐021, a dual endothelin receptor and angiotensin receptor blocker, in patients with focal segmental glomerulosclerosis (FSGS): a randomized, double‐blind, active‐control, dose‐escalation study. www.clinicaltrials.gov/ct2/show/NCT01613118 (accessed 2 March 2016).
-
- Basu B. Efficacy and safety of rituximab to that of calcineurin inhibitors in children with steroid resistant nephrotic syndrome. www.clinicaltrials.gov/ct2/show/NCT023825752015; Vol. (accessed 2 March 2016).
-
- Ghiggeri GM. Ofatumumab in children with steroid‐ and calcineurin‐inhibitor‐resistant nephrotic syndrome: a double‐blind randomized, controlled, superiority trial. www.clinicaltrials.gov/ct2/show/NCT023941062015; Vol. (accessed 2 March 2016).
Additional references
-
- Chua A, Yorgin P. Steroid‐Resistant Nephrotic Syndrome. In: Molony DA, Craig JC editor(s). Evidence‐Based Nephrology. Wiley‐Blackwell, 2009.
-
- Colquitt JL, Kirby J, Green C, Cooper K, Trompeter RS. The clinical effectiveness and cost‐effectiveness of treatments for children with idiopathic steroid‐resistant nephrotic syndrome: a systematic review. Health Technology Assessment (Winchester, England) 2007;11(21):1‐93. [MEDLINE: ] - PubMed
-
- Deegens JK, Wetzels JF. Immunosuppressive treatment of focal and segmental glomerulosclerosis: lessons from a randomized controlled trial. Kidney International 2011;80(8):798‐801. [MEDLINE: ] - PubMed
References to other published versions of this review
-
- Habashy D, Hodson EM, Craig JC. Interventions for steroid‐resistant nephrotic syndrome: a systematic review. Pediatric Nephrology 2003;18(9):906‐12. [MEDLINE: ] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

