Chronic opioid treatment augments caveolin-1 scaffolding: relevance to stimulatory μ-opioid receptor adenylyl cyclase signaling
- PMID: 27726130
- PMCID: PMC5123915
- DOI: 10.1111/jnc.13852
Chronic opioid treatment augments caveolin-1 scaffolding: relevance to stimulatory μ-opioid receptor adenylyl cyclase signaling
Abstract
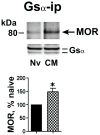
Caveolin-1 is the predominant structural protein of caveolae, a subset of (lipid) membrane rafts that compartmentalize cell signaling. Caveolin-1 binds most to G protein-coupled receptors and their signaling partners, thereby enhancing interactions among signaling cascade components and the relative activation of specific G protein-coupled pathways. This study reveals that chronic opioid exposure of μ-opioid receptor (MOR) expressing Chinese hamster ovary cells (MOR-CHO) and chronic in vivo morphine exposure of rat spinal cord augmented recruitment of multiple components of MOR-adenylyl cyclase (AC) stimulatory signaling by caveolin-1. Strikingly, in MOR-CHO and spinal cord, blocking the caveolin-1 scaffolding domain substantially attenuated the chronic morphine-induced increased interaction of caveolin-1 with MOR, Gsα, protein phosphatase 2A (PP2A), and AC. Chronic morphine treatment also increased interactions among the above signaling proteins, thus enabling sufentanil to stimulate (rather than inhibit) cAMP production within lipid membrane microdomains. The latter finding underscores the functionality of augmented interactions among MOR, Gs α, PP2A, and AC. In the aggregate, our data strongly suggest that augmented caveolin-1 scaffolding undergirds the ability of chronic opioids to recruit an ancillary signaling pathway by acting as an organizing template for MOR-Gs α-AC signaling and delimiting the membrane compartment(s) in which it occurs. Since caveolin-1 binds to a wide spectrum of signaling molecules, altered caveolin-1 scaffolding following chronic opioid treatment is likely to pertain to most, if not all, MOR signaling partners. The chronic morphine-induced trigger that augments caveolin-1 scaffolding could represent a seminal perturbation that initiates the wide spectrum of adaptations thought to contribute to opioid tolerance and dependence.
Keywords: caveolae; caveolin-1; opioid tolerance; opioids; μ-opioid receptor.
© 2016 International Society for Neurochemistry.
Conflict of interest statement
discloser The authors declare that they have no competing interests. Conflicts of interest: none
Figures



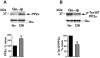


References
-
- Alvarez R, Daniels DV. A single column method for the assay of adenylate cyclase. Anal Biochem. 1990;187:98–103. - PubMed
-
- Bhatnagar A, Sheffler DJ, Kroeze WK, Compton-Toth B, Roth BL. Caveolin-1 interacts with 5-HT2A serotonin receptors and profoundly modulates the signaling of selected Galphaq-coupled protein receptors. J Biol Chem. 2004;279:34614–34623. - PubMed
-
- Boscher C, Nabi IR. Caveolin-1: role in cell signaling. Advances in experimental medicine and biology. 2012;729:29–50. - PubMed
-
- Bourova L, Kostrnova A, Hejnova L, Moravcova Z, Moon HE, Novotny J, Milligan G, Svoboda P. delta-Opioid receptors exhibit high efficiency when activating trimeric G proteins in membrane domains. J Neurochem. 2003;85:34–49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

