S-adenosyl methionine (SAMe) for depression in adults
- PMID: 27727432
- PMCID: PMC6457972
- DOI: 10.1002/14651858.CD011286.pub2
S-adenosyl methionine (SAMe) for depression in adults
Abstract
Background: Depression is a recurrent illness with high rates of chronicity, treatment-resistance and significant economic impact. There is evidence in the literature that S-adenosyl methionine (SAMe), a naturally occurring compound in the human body, has antidepressant efficacy. This product may be an important addition to the armamentarium of antidepressant agents.
Objectives: To assess the effects of SAMe in comparison with placebo or antidepressants for the treatment of depression in adults.
Search methods: We searched the Cochrane Common Mental Disorders Group's Specialised Register (CCMDCTR Studies and Reference Register), MEDLINE, EMBASE, PsycINFO, international trial registers ClinicalTrials.gov and the World Health Organization trials portal (ICTRP). We checked reference lists, performed handsearching and contacted experts in the field. The CCMDCTR literature search was last updated on 5 February 2016.
Selection criteria: Randomised controlled trials comparing SAMe with placebo or antidepressants in adults with a diagnosis of major depression.
Data collection and analysis: Two authors independently performed extraction of data and assessment of risk of bias. We contacted trialists of included studies for additional information.
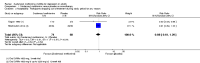
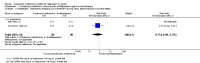
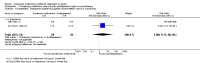
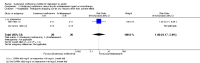
Main results: This systematic review included eight trials comparing SAMe with either placebo, imipramine, desipramine or escitalopram. We accepted trials that used SAMe as monotherapy or as add-on therapy to selective serotonin reuptake inhibitors (SSRIs), and we accepted both oral and parenteral administration. The review involved 934 adults, of both sexes, from inpatient and outpatient settings.The trials were at low risk of reporting bias. We judged the risk of selection, performance, detection and attrition bias as unclear or low, and one study was at high risk of attrition bias.There was no strong evidence of a difference in terms of change in depressive symptoms from baseline to end of treatment between SAMe and placebo as monotherapy (standardised mean difference (SMD) -0.54, 95% confidence interval (CI) -1.54 to 0.46; P = 0.29; 142 participants; 2 studies; very low quality evidence). There was also no strong evidence of a difference in terms of drop-out rates due to any reason between SAMe and placebo, when used as monotherapy (risk ratio (RR) 0.88, 95% CI 0.61 to 1.29; P = 0.52; 142 participants; 2 studies; low quality evidence).Low quality evidence showed that the change in depressive symptoms from baseline to end of treatment was similar between SAMe and imipramine, both as monotherapy (SMD -0.04, 95% CI -0.34 to 0.27; P = 0.82; 619 participants; 4 studies). There was also no strong evidence of a difference between SAMe and a tricyclic antidepressant in terms of drop-outs due to any reason (RR 0.61, 95% CI 0.28 to 1.31; P = 0.2; 78 participants; 3 studies; very low quality evidence).There was little evidence of a difference in terms of change in depressive symptoms from baseline to end of treatment between SAMe and escitalopram, both as monotherapy (MD 0.12, 95% CI -2.75 to 2.99; P = 0.93; 129 participants; 1 study; low quality evidence). There was no strong evidence of a difference between SAMe and escitalopram in terms of drop-outs due to any reason (RR 0.81, 95% CI 0.57 to 1.16; P = 0.26; 129 participants; 1 study; low quality evidence).There was low quality evidence that SAMe is superior to placebo as add-on to SSRIs in terms of change in depressive symptoms from baseline to end of treatment (MD -3.90, 95% CI -6.93 to -0.87; P = 0.01; 73 participants; 1 study). There was no strong evidence of a difference between SAMe and placebo as adjunctive therapy to an SSRI in terms of drop-outs due to any reason (RR 0.70, 95% CI 0.31 to 1.56; P = 0.38; 73 participants; 1 study; very low quality evidence).For all comparisons, secondary outcome measures of response and remission rates were consistent with these primary outcome measures.With regard to all extractable measures of the acceptability of SAMe, the quality of the evidence was low to very low. SAMe was not different from placebo and established antidepressants. The exception was that compared to imipramine, fewer participants experienced troublesome adverse effects when treated with parenteral SAMe.The specific adverse effects were not detailed in most of the included studies. There were two reports of mania/hypomania recorded for 441 participants in the SAMe arm.
Authors' conclusions: Given the absence of high quality evidence and the inability to draw firm conclusions based on that evidence, the use of SAMe for the treatment of depression in adults should be investigated further. Future trials should be in the form of large randomised controlled clinical trials of high methodological quality, with particular attention given to randomisation, allocation concealment, blinding and the handling of missing data. Comparator antidepressants from all classes should be used. Adverse events should be detailed for each participant, bearing in mind that induction of mania is of particular interest.
Conflict of interest statement
Dr Karine Macritchie has no conflicts of interest relevant to this review, but has attended educational symposia and conferences sponsored by several pharmaceutical companies. She has received a non‐promotional educational grant from Sanofi‐Synthélabo to support research. She has been employed at the University of Edinburgh on an award from the Translational Medicine Research Collaboration ‐ a consortium made up by the Universities of Aberdeen, Dundee, Edinburgh and Glasgow, the four associated National Health Service (NHS) Health Boards, Scottish Enterprise and Pfizer (formerly Wyeth).
Dr Ilaria Galizia, Dr Lucio Oldani, Ms Erica Amari, Dr Jacopo Massei, Dr Dominic Dougall and Dr Tessa Jones have no conflicts of interest to declare.
Dr Lam has been a speaker for, sat on advisory boards for, or has received research funds from: Advanced Neuromodulation Systems Inc, AstraZeneca, BrainCells Inc, Biovail, Canadian Institutes of Health Research, Canadian Network for Mood and Anxiety Treatments, Canadian Psychiatric Research Foundation, Eli Lilly, Janssen, Litebook Company Ltd, Lundbeck, Lundbeck Institute, Mathematics of Information Technology and Advanced Computing Systems, Michael Smith Foundation for Health Research, Servier, Takeda, UBC Institute of Mental Health/Coast Capital Savings and Wyeth.
Dr Lakshmi Yatham has received grants from and has been a speaker or member of advisory boards for AstraZeneca, Lilly, Janssen, Bristol Myers Squibb, Pfizer, Novartis, GlaxoSmithKline (GSK), Sanofi, Servier and Scherring Plough.
Professor Allan Young has no conflicts of interest relevant to this review, but he has received honoraria for lectures from Eli Lilly, AstraZeneca, Sanofi, GSK, Sanofi‐Aventis, Pfizer, Janssen, Servier, BMS, Biovail and Wyeth. He has been a member of advisory boards for Eli Lilly, AstraZeneca, Sanofi, GSK, Sanofi‐Aventis, Pfizer, Janssen, Servier, BMS and Wyeth. He has received research support from SMRI, MRC (UK), CIHR, NIH, Wellcome Trust (UK) and the following foundations: the University of British Columbia‐Vancouver General Hospital Foundations, BC Credit Unions, CoastCapital Savings and the Kelty Patrick Dennehy Foundation.
Figures



























































Update of
References
References to studies included in this review
Bell 1988 {published data only}
-
- Bell KM, Plon L, Bunney WE Jr, Potkin SG. S‐adenosylmethionine treatment of depression: a controlled clinical trial. American Journal of Psychiatry 1988;145(9):1110‐14. - PubMed
-
- Potkin SG, Bell K, Plon L, Bunney WE Jr. Rapid antidepressant response with SAMe. A double‐study. Alabama Journal of Medical Sciences 1988;25(3):313‐6. - PubMed
Bell 1994 {published data only}
-
- Bell KM, Carreon D, Plon L, Bunney We Jr, Potkin SG. Oral S‐adenosylmethionine in the treatment of depression: a double‐blind comparison with desipramine. Study report. BioResearch File 1990:53‐4.
-
- Bell KM, Potkin SG, Carreon D, Plon L. S‐adenosylmethionine blood levels in major depression: changes with drug treatment. Acta Neurologica Scandinavica 1994;154(Suppl 1):15‐8. - PubMed
Delle Chiaie 2000a {published data only}
-
- Delle Chiaie R, Pancheri P, Scapicchio P. Efficacy and tolerability of oral and intramuscular s‐adenosyl‐l‐methionine 1,4‐butanedisulfonate (SAMe) in the treatment of major depression: comparison with imipramine in 2 multicentrum studies. American Journal of Clinical Nutrition 2002;76(5):1172‐6. - PubMed
-
- Delle Chiaie R, Pancheri P, Scapicchio P. Mc3: multicentre, controlled efficacy and safety trial of oral S‐adenosyl‐methionine (SAMe) vs oral imipramine in the treatment of depression. International Journal of Neuropsychopharmacology 2000;3(Suppl 1):S230.
-
- Delle Chiaie R, Pancheri P, Scapicchio P. Multicentre, controlled efficacy and safety trial of S‐adenosyl‐methionine (SAMe) vs oral imipramine in the treatment of depression. International Journal of Neuropsychopharmacology 2000;3(Suppl 1):230.
-
- Delle Chiaie R, Pancheri P, Scapicchio P. S‐adenosyl‐methionine (SAMe) in major depression: two multicenter trials versus imipramine. 155th Annual Meeting of the American Psychiatric Association; 2002 May 18‐23; Philadelphia, PA. 2002.
-
- Delle Chiaie R, Pancheri R, Scapicchio P. S‐adenosyl‐methionine (SAMe) in major depression: two multicenter trials versus imipramine. 154th Annual Meeting of the American Psychiatric Association; 2001 May 5‐10, New Orleans. 2000:NR499.
Delle Chiaie 2000b {published data only}
-
- Delle Chiaie R, Pancheri P, Scapicchio P. Efficacy and tolerability of oral and intramuscular s‐adenosyl‐l‐methionine 1,4‐butanedisulfunate (SAMe) in the treatment of major depression: comparison with imipramine in 2 multicenter studies. American Journal of Clinical Nutrition 2002;76(5):1172‐6. - PubMed
-
- Delle Chiaie R, Pancheri P, Scapicchio P. Mc4: multicentre, controlled efficacy and safety trial of intramuscular S‐adenosylmethionine (SAMe) versus oral imipramine in the treatment of depression. International Journal of Neuropsychopharmacology 2000;3(Suppl 1):S230.
-
- Delle Chiaie R, Pancheri P, Scapicchio P. S‐adenosyl‐methionine (SAMe) in major depression: two multicenter trials versus imipramine. 154th Annual Meeting of the American Psychiatric Association; 2001 May 5‐10, New Orleans. 2001:NR499.
-
- Delle Chiaie R, Pancheri P, Scapicchio P. S‐adenosyl‐methionine (SAMe) in major depression: two multicenter trials versus imipramine. 155th Annual Meeting of the American Psychiatric Association; 2002 May 18‐23; Philadelphia, PA. 2002.
-
- Pancheri P, Scapicchio P, Delle Chiaie R. A double‐blind, randomized parallel‐group, efficacy and safety study of intramuscular S‐adenosyl‐L‐methionine 1,4‐butanedisulphonate (SAMe) versus imipramine in patients with major depressive disorder. International Journal of Neuropsychopharmacology 2002;5(4):287‐94. - PubMed
De Vanna 1992 {published data only}
-
- Vanna M, Rigamonti R. Oral S‐adenosyl‐l‐methionine in depression. Current Therapeutic Research, Clinical and Experimental 1992;52(3):478‐85.
Kagan 1990 {published data only}
-
- Kagan BL, Sultzer L, Rosenlicht N, Gerner RH. Oral S‐adenosylmethionine in depression: a randomized, double‐blind, placebo‐controlled trial. American Journal of Psychiatry 1990;147(5):591‐5. - PubMed
Mischoulon 2014 {published data only}
-
- Fava M. A double‐blind, placebo‐controlled study of the alternative therapy S‐adenosyl‐l‐methionine (SAMe) versus escitalopram in major depressive disorder. clinicaltrials.gov/show/NCT00101452 (accessed 3 August 2016). [http://clinicaltrials.gov/show/NCT00101452]
-
- Gerbarg PL, Muskin PR, Bottiglieri T, Brown RP. Failed studies should not be used to malign good treatments [NCT00101452]. Journal of Clinical Psychiatry 2014;75(11):e1328. - PubMed
-
- Mischoulon D, Price LH, Carpenter LL, Tyrka AR, Papakostas GI, Fava M. Dr. Mischoulon and colleagues reply [NCT00101452]. Comment on failed studies should not be used to malign good treatments. Journal of Clinical Psychiatry 2014;75(11):e1328‐9. - PubMed
-
- Sarris J, Papakostas GI, Vitolo O, Fava M, Mischoulon D. S‐adenosylmethionine (SAMe) versus escitalopram and placebo in major depression RCT: efficacy and effects of histamine and carnitine as moderators of response. Journal of Affective Disorders 2014;164:76‐81. - PubMed
Papakostas 2010a {published data only}
-
- Mischoulon D, Alpert JE, Arning E, Bottiglieri T, Fava M, Papakostas GI. Bioavailability of S‐adenosyl methionine and impact on response in a randomized, double‐blind, placebo‐controlled trial in major depressive disorder [NCT00093847]. Journal of Clinical Psychiatry 2012;73(6):843‐8. - PMC - PubMed
-
- Papakostas GI. S‐Adenosyl methionine (SAMe) augmentation of selective serotonin reuptake inhibitors (SSRIs) for treatment‐resistant depression (TRD). clinicaltrials.gov/show/NCT00093847 (accessed 3 August 2016). [http://clinicaltrials.gov/show/NCT00093847]
-
- Papakostas GI, Mischoulon D, Shyu I, Alpert JE, Fava M. S‐adenosyl methionine (SAMe) augmentation of serotonin reuptake inhibitors for antidepressant nonresponders with major depressive disorder: a double‐blind, randomized clinical trial. American Journal of Psychiatry 2010;167(8):942‐8. - PubMed
References to studies excluded from this review
Agnoli 1975 {published data only}
-
- Agnoli A, Fazio C, Andreoli V. Neuropsychiatric disorders and transmethylations: therapeutic effects of S adenosyl L methionine [Disturbi neuropsichiatrici e transmetilazioni: effetti terapeutici della S adenosil l metionina]. Clinica Terapeutica 1975;75(6):567‐79. - PubMed
Agnoli 1976 {published data only}
-
- Agnoli A, Andreoli V, Casacchia M, Cerbo R. Effect of S‐adenosyl‐l‐methionine (SAMe) upon depressive symptoms. Journal of Psychiatric Research 1976;13(1):43‐54. - PubMed
Agnoli 1978 {published data only}
-
- Agnoli A, Andreoli VM, Casacchia M, Maffei F, Fazio C. Results and possible developments of the clinical use of S‐adenosyl‐L‐methionine (SAMe) in psychiatry. Monographien aus dem Gesamtgebiete der Psychiatrie 1978;18:170‐82. - PubMed
Alvarez 1984 {published data only}
-
- Alvarez E, Udina C, Guillamat R. Double blind study on the antidepressant activity of sulfoadenosyl‐L‐methionine (SAMe): clinical and biological factors [Estudio doble ciego sobre la efectividad antidepresiva del SAMe; factores clínicos y biológicos]. Revista del Departamento de Psiquiatría de la Facultad de Medicina de Barcelona 1984;11(4):275‐88.
Bambling 2015 {published data only}
-
- Bambling M, Parham SC, Coulson S, Vitetta L. S‐adenosylmethionine (SAMe) and magnesium orotate as adjunctives to SSRIs in sub‐optimal treatment response of depression in adults: a pilot study. Advances in Integrative Medicine 2015;2(1):56‐62.
Barberi 1978 {published data only}
-
- Barberi A, Pusateri C. The clinical effects of S‐adenosyl‐L‐methionine (SAMe) in cases of depression [Sugli effetti clinici della S‐adenosil‐L‐metionina (SAMe) nelle sindromi depressive]. Minerva Psichiatrica 1978;19(4):235‐43.
Berlanga 1992 {published data only}
-
- Berlanga C, Ortega Soto HA, Ontiveros M, Senties H. Efficacy of S‐adenosyl‐L‐methionine in speeding the onset of action of imipramine. Psychiatry Research 1992;44(3):257‐62. - PubMed
Blasi Ras 1985 {published data only}
-
- Blasi Ras R. Comparative study of sulfoadenosyl‐l‐methionine and amoxapine in depression [Estudio comparativo sulfoadenosil‐l‐metionina con amoxapina en la patologia depresiva]. Psiquis, Revista de Psiquiatria, Psicologia y Psicosomatica 1985;6(4):35‐44.
Bottiglieri 1986 {published data only}
-
- Bottiglieri T, Carney MWP, Edeh J, Laundy M, Martin R, Reynolds EH, et al. A biochemical study of depressed patients receiving S‐adenosyl‐L‐methionine (SAM). Biological Methylation and Drug Design. Experimental Biology and Medicine 1986;12:327‐38.
Bottiglieri 1990 {published data only}
Calandra 1979 {published data only}
-
- Calandra C, Roxas M, Rapisarda V. The antidepressant action of SAMe compared with that of chloripramine and an interpretation of its mechanism [Azione antidepressiva della SAMe a paragone della clorimipramina. Ipotesi interpretative del meccanismo d’azione]. Minerva Psichiatrica 1979;20(2):147‐52. - PubMed
Carney 1986 {published data only}
-
- Carney MW, Edeh J, Bottiglieri T, Reynolds EM, Toone BK. Affective illness and S adenosylmethionine: a preliminary report. Clinical Neuropharmacology 1986;9(4):379‐85. - PubMed
De Leo 1987 {published data only}
-
- Leo D. S‐adenosylmethionine as an antidepressant: a double‐blind trial versus placebo. Current Therapeutic Research, Clinical and Experimental 1987;41(6):865‐70.
Delle Chiaie 1999 {published data only}
-
- Delle Chiaie R, Boissard G. Meta‐analysis of two European multicenter controlled trials with ademetionine (SAMe) in major depression. 6th World Congress of Biological Psychiatry; 1997 22‐27 Jun; Nice, France. 1997.
-
- Delle Chiaie R, Pancheri P. Combined analysis of two controlled, multicentric, double‐blind studies to assess efficacy and safety of sulfo‐adenosyl‐methionine (SAMe) vs. placebo (MC1) and SAMe vs. clomipramine (MC2) in the treatment of major depression [Analisi combinata di due studi controllati, multicentrici, in doppio cieco per la valutazione di efficacia e tollerabilita' di SAMe vs. placebo (MC1) e di SAMe vs. clomipramina (MC2) nel trattamento della depressione maggiore]. Giornale Italiano di Psicopatologia 1999;5:1‐16.
Del Vecchio 1978 {published data only}
-
- Vecchio M, Iorio G, Cocurullo M, Vacca L, Amati A. Has SAMe (Ado Met) and antidepressant effect: a preliminary trial versus chlorimipramine. Rivista Sperimentale di Freniatria 1978;102:344‐58.
Di Pierro 2015 {published data only}
Fava 1992 {published data only}
-
- Fava M, Rosenbaum JF, Birnbaum R, Kelly K, Otto MW, MacLaughlin R. The thyrotropin response to thyrotropin‐releasing hormone as a predictor of response to treatment in depressed outpatients. Acta Psychiatrica Scandinavica 1992;86(1):42‐5. - PubMed
Janicak 1988 {published data only}
-
- Janicak PG, Lipinski J, Davis JM, Altman E, Sharma RP. Parenteral S‐adenosylmethionine (SAMe) in depression; literature review and preliminary data. Psychopharmacology Bulletin 1989;25(2):238‐42. - PubMed
-
- Janicak PG, Lipinski J, Davis JM, Comaty JE, Waternaux C, Cohen B, et al. S‐adenosylmethionine in depression. A literature review and preliminary report. Alabama Journal of Medical Sciences 1988;25(3):306‐13. - PubMed
Kufferle 1982 {published data only}
-
- Kufferle B, Grunberger J. Early clinical double‐blind study with S‐adenosyl‐L‐methionine: a new potential antidepressant. Advances in Biochemical Psychopharmacology 1982;32:175‐80. - PubMed
Lanaia 1977 {published data only}
-
- Lanaia F, Savoca G, Zappala E, Magro E, Calandra A. SAMe in depressive syndromes [La SAMe nelle sindromi depressive]. Igiene Mentale 1977;2:397‐406.
Mantero 1976 {published data only}
-
- Mantero M, Pastorino P. Depressive syndromes, skin disease and transmethylations. Therapeutic effects of S adenosyl L methionine [Sindromi depressive, malattie cutanee e transmetilazioni. Effetti terapeutici della S adenosil L metionina]. Gazzetta Medica Italiana 1976;135(12):707‐16.
Muscettola 1982 {published data only}
-
- Muscettola G, Galzenati M, Balbi A. SAMe versus placebo: a double blind comparison in major depressive disorders. Advances in Biochemical Psychopharmacology 1982;32:151‐6. - PubMed
Rabassini 1979 {published data only}
-
- Rabassini A, Russo G. Effects of the association of maprotiline + SAMe in depressive syndromes [Effetti dell’associazione maprotilina + SAMe nelle sindromi depressive]. Psichiatria Generale e Dell'eta' Evolutiva 1979;3:305‐23.
Salmaggi 1991 {published data only}
-
- Salmaggi P, Bressa GM, Nicchia G, Coniglio M, Greca P, Grazie C. Double‐blind, placebo‐controlled study of S‐adenosyl‐L‐methionine in depressed postmenopausal women. Psychotherapy and Psychosomatics 1993;59(1):34‐40. - PubMed
-
- Salmaggi P, Bressa GM, Niccia G, Coniglio M, Greca P, Grazie C. Oral S‐adenosylmethionine (SAMe) in the treatment of menopausal depressive disorder (MDD): a double‐blind placebo controlled trial. European Journal of Clinical Investigation 1991;21(2 pt 2):58.
Sarris 2015b {published data only}
-
- Sarris J, Stough C, Bousman C, Murphy J, Savage K, Smith DJ, et al. An adjunctive antidepressant nutraceutical combination in treating major depression: study protocol, and clinical considerations. Advances in Integrative Medicine 2015;2(1):49‐55.
Scarzella 1978 {published data only}
-
- Scarzella R, Appiotti A. A double‐blind clinical comparison of SAMe vs clomipramine in depressive disorders [Confronto clinico in doppio cieco della SAMe versus clorimipramina nelle sindromi depressive]. Rivista Sperimentale di Freniatria 1978;102:359‐65.
Schifano 1993 {published data only}
-
- Schifano F, Garofoli F. Dothiepin vs S‐adenosylmethionine (SAMe) for the treatment of major depression in weaned alcoholics: a pilot study. European Neuropsychopharmacology 1993;3(3):330.
Thomas 1987 {published data only}
-
- Thomas CS, Bottiglieri T, Edeh J, Carney MW, Reynolds EH, Toone BK. The influence of S‐adenosylmethionine (SAM) on prolactin in depressed patients. International Clinical Psychopharmacology 1987;2(2):97‐102. - PubMed
References to studies awaiting assessment
Quiros 1982 {published data only}
-
- Quiros G. Assessment of a new drug for depression: S‐adenosyl‐L‐methionine [Valoracion de un nuevo farmaco en el tratamiento de las depresiones: la S‐Adenosyl‐L‐Metionina]. Psicopatologia 1982;2(3):265‐74.
References to ongoing studies
ACTRN12613001299796 {published data only}
-
- Sarris J. Nutraceuticals as monotherapy treatments in major depressive disorder: a double‐blind, randomised, placebo‐controlled trial, 2013. www.anzctr.org.au/ACTRN12613001299796.aspx (accessed 3 August 2016). [www.anzctr.org.au/ACTRN12613001299796.aspx]
-
- Sarris J, Murphy J. The efficacy of S‐adenosyl methionine (SAMe) and a combination nutraceutical as monotherapy treatments in major depressive disorder: a double‐blind, randomised, placebo‐controlled trial. www.anzctr.org.au/ACTRN12613001299796.aspx (accessed 3 August 2016).
ACTRN12613001300763 {published data only}
-
- Sarris J. The efficacy of S‐adenosylmethionine (SAMe) and a combination nutraceutical as adjunctive treatments in depression: a double‐blind, randomized, placebo‐controlled trial, 2013. www.anzctr.org.au/ACTRN12613001300763.aspx (accessed 3 August 2016). [www.anzctr.org.au/ACTRN12613001300763.aspx]
NCT01912196 {published data only}
-
- MSI Methylation Sciences, Inc. A double‐blind, placebo‐controlled, randomized add‐on study of MSI‐195 (Methylation Sciences Inc. S‐adenosyl‐L‐methionine, SAMe) for patients with major depressive disorder (MDD) who have had an inadequate response to current antidepressant therapy, 2013. clinicaltrials.gov/show/NCT01912196 (accessed 3 August 2016). [clinicaltrials.gov/show/NCT01912196]
Additional references
Alderson 2004
-
- Alderson P, Green S, Higgins JPT. Cochrane Reviewers' Handbook 4.2.2 [updated December 2003]. 4.2.2. Chichester, UK: Wiley & Sons, Ltd, 2004.
Algeri 1979
-
- Algeri S, Catto E, Curcio M, Ponzi F, Stamentinoli G. Changes in rat brain noradrenaline and serotonin after administration of S‐adenosylmethionine. In: Zappia V, Usdin E, Salvatore F editor(s). Biochemical and Pharmacological Roles of Adenosylmethionine and the Central Nervous System. New York: Permagon Press, 1979:81‐7.
Altman 1996a
Altman 1996b
Alvarez 1987
-
- Alvarez E, Udina C, Guillamat R. Shortening of latency period in depressed patients treated with SAMe and other antidepressant drugs. Cell Biology Reviews 1987;S1:103‐10.
APA 1980
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Third Edition (DSM III). Washington DC: American Psychiatric Association, 1980.
APA 1994
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM‐IV). Washington DC: American Psychiatric Association, 1994.
APA 2000
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. Washington, DC: American Psychiatric Association, 2000.
APA 2010
-
- American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder, 3rd edition. 2010. - PubMed
Arroll 2009
Bell (personal communication)
-
- Bell KM, Potkin SG, Carreon L, Plot L, Bunney W. Oral S‐adenosylmethionine in the treatment of depression: a double‐blind, randomized comparison with desipramine. American Journal Psychiatry (submitted).
Bell 1987
-
- Bell K, Plon L, Nabel M. A controlled clinical trial of S‐adenosylmethionine treatment of depression. Annual Review of Cell and Developmental Biology 1987;S1:111‐20.
Berlim 2007
-
- Berlim MT, Turecki G. Definition, assessment, and staging of treatment‐resistant refractory major depression: a review of current concepts and methods. Canadian Journal of Psychiatry 2007;52:46‐54. - PubMed
Bland 1997
BNF 2016
-
- Royal Pharmaceutical Society of Great Britain, British Medical Association. British National Formulary. London: British Medical Association, September 2016.
Bottiglieri 2002
-
- Bottiglieri T. S‐adenosyl‐L‐methionine (SAMe): from the bench to the bedside ‐ molecular basis of a pleiotrophic molecule. American Journal of Clinical Nutrition 2002;78:1151S‐7S. - PubMed
Bressa 1994
-
- Bressa GM. S‐adenosyl‐l‐methionine (SAMe) as antidepressant: meta‐analysis of clinical studies. Acta Neurologica Scandinavica. Supplementum 1994;154:7‐14. - PubMed
Byford 2011
Cantoni 1952
-
- Cantoni GL. The nature of the active methyl donor formed enzymatically from L‐methionine and adenosine triphosphate. Journal of the American Chemical Society 1952;74(11):2942‐3.
Carney 1989
-
- Carney MW, Chary TK, Bottiglieri T, Reynolds EH. The switch mechanism and the bipolar/unipolar dichotomy. British Journal of Psychiatry 1989;154:48‐51. - PubMed
Carpenter 2011
-
- Carpenter DJ. St. John's wort and S‐adenosyl methionine as "natural" alternatives to conventional antidepressants in the era of the suicidality boxed warning: what is the evidence for clinically relevant benefit?. Alternative Medicine Review 2011;16(1):17‐39. - PubMed
Chavez 2000
-
- Chavez M. SAMe: S‐Adenosylmethionine. American Journal of Health‐System Pharmacy 2000;57(2):119‐23. - PubMed
Craig 2007
-
- Craig D, Rice S. NHS Economic Evaluation Database Handbook. York, UK: Centre for Reviews and Dissemination, University of York, 2007.
Craig Nelson 2010
-
- Craig Nelson J. S‐Adenosyl Methionine (SAMe) augmentation in major depressive disorder. American Journal of Psychiatry 2010;167:889‐91. [10.1176/appi.ajp.2010.10040627] - PubMed
Curcio 1978
-
- Curcio M, Catto E, Stramentinoli G, Algeri S. Effect of S‐adenosyl‐L‐methionine on serotonin metabolism in rat brain. Progress in Neuro‐Psychopharmacology 1978;2(1):65‐71. - PubMed
Davidson 2010
Delle Chiaie 2002
-
- Delle Chiaie R, Pancheri P, Scapicchio P. Efficacy and tolerability of oral and intramuscular S‐adenosyl‐L‐methionine 1,4‐butanedisulfonate (SAMe) in the treatment of major depression: comparison with imipramine in 2 multicenter studies. American Journal of Clinical Nutrition 2002;76(5) :1172S‐6S. - PubMed
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in Clinical Trials. Controlled Clinical Trials 1986;7:177‐88. - PubMed
Di Padova 2000
-
- Padova C, Giudici A, Boissard G. Ademetionine and depression. IV Workshop on methionine metabolism: molecular mechanisms and clinical implications; 2000 Feb 20‐24; Granada, Spain. 2000:295‐9.
Di Rocco 2000
-
- Rocco A, Rogers JD, Brown R, Werner P, Bottiglieri T. S‐Adenosyl methionine improves depression in patients with Parkinson's disease in an open‐label clinical trial. Movement Disorders 2000;15 (6):1225‐9. - PubMed
Divine 1992
-
- Divine GW, Brown JT, Frazer LM. The unit of analysis error in studies about physicians' patient care behavior. Journal of General Internal Medicine 1992;7:623‐9. - PubMed
Drummond 1996
Fawcett 1983
-
- Fawcett J, Kravitz HM. Anxiety syndromes and their relationship to depressive illness. Journal of Clinical Psychiatry 1983;44(8, Pt2):8‐11. - PubMed
Fazio 1974
-
- Fazio C, Andreoli V, Agnoli A, Casacchia M, Cerbo R. Therapy of schizophrenic and depressive disorders with S‐adenosylmethionine. Clinical Pharmacology & Therapeutics 1974;2:1015.
Fleisch 2010
-
- Fleisch SB, Travis MJ, Ryan ND. Discrepancy in response and remission rates for SAMe‐treated patients in a double‐blind, randomized clinical trial [comment]. American Journal of Psychiatry 2010;167(12):1533. - PubMed
Furukawa 2006
-
- Furukawa TA, Barbui C, Cipriani A, Brambillac P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59:7‐10. - PubMed
Goldberg 2012
-
- Goldberg D, Fawcett J. The importance of anxiety in both major depression and bipolar disorder. Depression and Anxiety 2012;29(6):471‐8. - PubMed
Greenberg 2003
-
- Greenberg PE, Kessler RC, Birnbaum HG, Leong SA, Lowe SW, Berglund PA, et al. The economic burden of depression in the United States: how did it change between 1990 and 2000?. Journal of Clinical Psychiatry 2003;64(12):1465‐75. - PubMed
Gulliford 1999
-
- Gulliford MC, Ukoumunne OC, Chinn S. Components of variance and intraclass correlations for the design of community‐based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology 1999;149:876‐83. - PubMed
Gören 2004
-
- Gören JL, Stoll AL, Damico KE, Sarmiento IA, Cohen BM. Bioavailability and lack of toxicity of S‐adenosyl‐L‐methionine (SAMe) in humans. Pharmacotherapy 2004;24(11):1501‐7. - PubMed
Hamilton 1960
Hardy 2002
-
- Hardy M, Coulter I, Morton SC, Favreau J, Venuturupalli S, Chiappelli F, et al. S‐adenosyl‐L‐methionine for treatment of depression, osteoarthritis, and liver disease. Evidence Report/Technology Assessment Number 64. Rockville, MD: Agency for Healthcare Research and Quality October 2002. - PMC - PubMed
Henkel 2006
-
- Henkel V, Mergi R, Allgaier AK, Kohnen R, Möller HJ, Hegeri U. Treatment of depression with atypical features: a meta‐analytic approach. Psychiatry Research 2006;141:89‐101. - PubMed
Higgins 2011a
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
Kessler 2012
King's Fund 2008
-
- McCrone P, Dhanasiri S, Pate A, Knapp M, Lawton‐Smith S. Paying the Price. The Cost of Mental Health Care in England to 2026. London: The King's Fund, 2008.
Kresge 2005
-
- Kresge N, Tabor H, Simoni RD, Hill RL. An escape from Italy, the discovery of S‐adenosylmethionine, and the biosynthesis of creatine by Giulio L. Cantoni. Journal of Biological Chemistry 2005;280:e35. - PubMed
Lipinski 1984
-
- Lipinski JF, Cohen BM, Frankenburg F, Tohen M, Waternaux C, Altesman R, Jones B. Open trial of S‐adenosylmethionine for treatment of depression. American Journal of Psychiatry 1984;141:448‐50. - PubMed
Macher (in press)
-
- Macher JR. A double‐blind, placebo‐controlled trial of intravenous ademetionine (SAMe) in the treatment of major depression (DSM III‐R). International Clinical Psychopharmacology (in press).
Macher 2000
-
- Macher JP. A double blind placebo controlled trial of intravenous ademetionine (SAMe) in the treatment of major depression. Progress in Neuro‐Psychopharmacology & Biological Psychiatry 2000;24(2):207‐25. - PubMed
Mischoulon 2002
-
- Mischoulon D, Fava M. Role of S‐adenosyl L‐methionine in the treatment of depression: a review of the evidence. American Journal of Clinical Nutrition 2002;76:1158S‐61S. - PubMed
Montgomery 1979
-
- Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry 1979;134:382‐9. - PubMed
Morelli 2000
-
- Morelli V, Zoorob RJ. Alternative therapies: Part I. Depression, diabetes, obesity. American Family Physician 2000;62(5):1051‐60. - PubMed
Murray 1997
-
- Murray CJL, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet 1997;349(9063):1436‐42. - PubMed
Nemeroff 2007
-
- Nemeroff CB. Prevalence and management of treatment‐resistant depression. Journal of Clinical Psychiatry 2007;68 Suppl 8:17‐25. - PubMed
NICE 2009
-
- National Institute for Health and Care Excellence. Depression in adults. The treatment and management of depression in adults. NICE clinical guideline 90 October 2009.
Otero‐Losado 1989a
-
- Otero‐Losado ME, Rubio MC. Acute effects of S‐adenosylmethionine on catecholaminergic central function. European Journal of Pharmacology 1989;163:353‐6. - PubMed
Otero‐Losado 1989b
-
- Otero‐Losado ME, Rubio MC. Acute changes in 5HT metabolism after S‐adenosylmethionine administration. General Pharmacology 1989;20:403‐6. - PubMed
Pancheri 1997
-
- Pancheri P, Racagni G, Delle Chiaie RD. Recent experimental and clinical findings of the efficacy and safety of adementionine (SAMe) in the pharmacological treatment of depression. Giornale Italiano di Psicopatologia 1997;3:1‐23.
Papakostas 2003
-
- Papakostas GI, Alpert JE, Fava M. S‐adenosyl methionine in the treatment of depression: a comprehensive review of the literature. Current Psychiatry Reports 2003;5:460‐6. - PubMed
Papakostas 2010b
-
- Papakostas GI. 'Discrepancy in response and remission rates for SAMe‐treated patients in a double‐blind, randomized clinical trial': reply to Fleisch et al [letter]. American Journal of Psychiatry 2010;167(12):1533. - PubMed
Pinzello 1972
-
- Pinzello A, Andreoli V. SAMe‐dependent transmethylation in the depressive syndromes: evaluation of the therapeutic effect of S‐adenosylmethionine using Hamilton Rating Scale [Le transmetilazioni SAM‐dipendenti nelle sindromi depressive. Valutazione dell'effetto terapeutico della S‐adenosilmetionina con la scala di Hamilton]. Quaderno Terapeutico Sperimentale; Supplemento Biochimica e Biol Sperimentale 1972;10(2):3‐11.
Posternak 2000
-
- Posternak MA, Zimmermann M. Short‐term spontaneous improvement rates in depressed outpatients. Journal of Nervous and Mental Disease 2000;188(12):799‐804. - PubMed
Rambaldi 2006
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rothwell 2005
-
- Rothwell PM. External validity of randomised controlled trials: to whom do the results apply?. Lancet 2005;365:82‐93. - PubMed
Sackett 1996
-
- Sackett DL, Deeks JJ, Altman DG. Down with odds ratios!. Evidence Based Medicine 1996;1:164‐6.
Sarris 2010
-
- Sarris J, Kavanagh DJ, Byrne G. Adjuvant use of nutritional and herbal medicines with antidepressants, mood stabilizers and benzodiazepines. Journal of Psychiatric Research 2010;44:32‐41. - PubMed
Sarris 2015a
Shippy 2004
Sinclair 1994
-
- Sinclair JC, Bracken MB. Clinically useful measures of effect in binary analyses of randomized trials. Journal of Clinical Epidemiology 1994;47:881‐9. - PubMed
Sobocki 2006
-
- Sobocki P, Jönsson B, Angst J, Rehnberg C. Cost of depression in Europe. Journal of Mental Health Policy and Economics 2006;9(2):87‐98. - PubMed
Stramentinoli 1979
-
- Stramentinoli G, Gualano M, Galli‐Kienzle M. Intestinal absorption of S‐adenosyl‐L‐methionine. Journal of Pharmacology and Experimental Therapeutics 1979;209:323‐6. - PubMed
Sugden 2006
-
- Sugden C. One‐carbon metabolism in psychiatric illness. Nutrition Research Reviews 2006;19:117‐36. - PubMed
WHO 1978
-
- World Health Organization. Mental disorders: glossary and guide to their classification in accordance with the ninth revision of the International Classification of Diseases. Geneva: WHO, 1978.
WHO 1992
-
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD‐10). Geneva: World Health Organization, 1992.
Williams 2005
-
- Williams AL, Girard C, Jui D, Sabina A, Katz Dl. S‐adenosylmethionine (SAMe) as treatment for depression: a systematic review. Clinical and Investigative Medicine 2005;28:132‐9. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

