Hydromorphone for cancer pain
- PMID: 27727452
- PMCID: PMC6457981
- DOI: 10.1002/14651858.CD011108.pub2
Hydromorphone for cancer pain
Update in
-
Hydromorphone for cancer pain.Cochrane Database Syst Rev. 2021 Aug 5;8(8):CD011108. doi: 10.1002/14651858.CD011108.pub3. Cochrane Database Syst Rev. 2021. PMID: 34350974 Free PMC article.
Abstract
Background: Cancer pain is an important and distressing symptom that tends to increase in frequency and intensity as the cancer advances. For people with advanced cancer, the prevalence of pain can be as high as 90%. It has been estimated that 30% to 50% of people with cancer categorise their pain as moderate to severe, with between 75% and 90% of people with cancer experiencing pain that they describe as having a major impact on their daily life. Epidemiological studies suggest that approximately 15% of people with cancer pain fail to experience acceptable pain relief with conventional management. Uncontrolled pain can lead to physical and psychological distress and can, consequently, have a drastic effect on people's quality of life.
Objectives: To determine the analgesic efficacy of hydromorphone in relieving cancer pain, as well as the incidence and severity of any adverse events.
Search methods: We searched the Cochrane Central Register of Controlled Trials, MEDLINE, Embase and clinical trials registers up to April 2016. There were no language, document type or publication status limitations applied in the search.
Selection criteria: We included randomised controlled trials (RCTs) that compared hydromorphone with placebo or other active pain medication for cancer pain in both adults and children. The four main outcomes selected have previously been identified as important to people with cancer; pain no worse than mild pain, and the impact of the treatment on consciousness, appetite and thirst. We did not consider physician-, nurse- or carer-reported measures of pain.
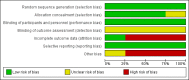
Data collection and analysis: Two review authors independently extracted data. For binary outcomes, we calculated risk ratio (RR) and its 95% confidence interval (CI), on an intention-to-treat basis. For continuous data, we estimated the mean difference (MD) between groups and its 95% CI. We used a random-effects model and assessed the risk of bias for all included studies. A meta-analysis was not completed on any of the primary outcomes in this review due to the lack of data. We assessed the evidence using GRADE and created two 'Summary of findings' tables.
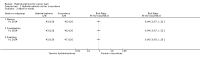
Main results: We included four studies (604 adult participants), which compared hydromorphone to oxycodone (two studies) or morphine (two studies). Overall, the included studies were at low or unclear risk of bias, rated unclear due to unknown status of blinding of outcome assessment; we rated three studies at high risk of bias for potential conflict of interest. Data for 504 participants were available for analysis. We collected data on endpoint participant-reported pain intensity measured with a visual analogue scale (VAS) (mean ± standard deviation (SD): hydromorphone 28.86 ± 17.08, n = 19; oxycodone 30.30 ± 25.33, n = 12; scale from 0 to 100 with higher score indicating worse pain), and Brief Pain Inventory (BPI) 24 hours worst pain subscale (mean ± SD: hydromorphone 3.5 ± 2.9, n = 99; morphine 4.3 ± 3.0, n = 101, scale from 0 to 10 with higher score indicating worse pain). The data demonstrated a similar effect between groups with both comparisons. The pain intensity data showed that participants in all four trials achieved no worse than mild pain. There were several adverse events: some were the expected opioid adverse effects such as nausea, constipation and vomiting; others were not typical opioid adverse effects (for example, decreased appetite, dizziness and pyrexia, as shown in Table 1 in the main review), but generally showed no difference between groups. There were three deaths in the morphine group during the trial period, considered to be due to disease progression and unrelated to the drug. Three trials had over 10% dropout, but the reason and proportion of dropout was balanced between groups. The overall quality of evidence was very low mainly due to high risk of bias, imprecision of effect estimates and publication bias. There were no data available for children or for several participant-important outcomes, including participant-reported pain relief and treatment impact on consciousness, appetite or thirst.
Authors' conclusions: This review indicated little difference between hydromorphone and other opioids in terms of analgesic efficacy. Data gathered in this review showed that hydromorphone had a similar effect on participant-reported pain intensity as reported for oxycodone and morphine. Participants generally achieved no worse than mild pain after taking hydromorphone, which is comparable with the other drugs. It produced a consistent analgesic effect through the night and could be considered for use in people with cancer pain experiencing sleep disturbance. However, the overall quality of evidence was very low mainly due to risk of bias, imprecision of effect estimates and publication bias. This review only included four studies with limited sample size and a range of study designs. Data for some important outcomes, such as impact of the treatment on consciousness, appetite or thirst, were not available. Therefore, we were unable to demonstrate superiority or inferiority of hydromorphone in comparison with other analgesics for these outcomes. We recommend that further research with larger sample sizes and more comprehensive outcome data collection is required.
Conflict of interest statement
YJB: none known; YJB is a specialist oncology physician and manages patients with cancer pain.
WH: none known; WH is a specialist oncology physician and manages patients with cancer pain.
XYK: none known; XYK is a pharmacologist.
LPY: none known; LPY is a specialist nephropathy physician and manages patients with nephropathy.
JX: none known; JX is a methodologist and does not have a clinical connection.
BJH: none known; BJH is a specialist oncology physician and manages patients with cancer pain.
RK: none known; RK is a pharmacist and manages patients with pain.
Figures
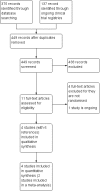






References
References to studies included in this review
-
- Hagen NA, Babul N. Comparative clinical efficacy and safety of a novel controlled‐release oxycodone formulation and controlled‐release hydromorphone in the treatment of cancer pain. Cancer1997; Vol. 79, issue 7:1428‐37. - PubMed
-
- Hanna M, Thipphawong J, 118 Study Group. A randomized, double‐blind comparison of OROS(R) hydromorphone and controlled‐release morphine for the control of chronic cancer pain. BMC palliative care 2008;7:17. - PMC - PubMed
- NCT00410540. A study of OROS hydromorphone HCl vs morphine in cancer pain patients [A randomized, double‐blind, controlled trial of hydromorphone (immediate and sustained‐ release) vs morphine (immediate and sustained‐release) in cancer pain]. clinicaltrials.gov/ct2/show/NCT00410540 First received: 12 December 2006.
-
- Moriarty M, McDonald CJ, Miller AJ. A randomised crossover comparison of controlled release hydromorphone tablets with controlled release morphine tablets in patients with cancer pain. Journal of Clinical Research1999; Vol. 2:1‐8.
-
- NCT01205126. An efficacy and safety study of oral osmotic therapeutic system (OROS) hydromorphone hydrochloride (HCl) in participants with cancer related pain [A randomized, double‐blind, active controlled, multi‐center study to investigate the safety and efficacy of OROS hydromorphone HCl once‐daily compared with oxycodone HCL controlled‐release twice daily in subjects with cancer pain]. clinicaltrials.gov/ct2/show/NCT01205126 First received: 5 August 2010.
- Yu S, Shen W, Yu L, Hou Y, Han J, Richards HM. Safety and efficacy of once‐daily hydromorphone extended‐release versus twice‐daily oxycodone hydrochloride controlled‐release in Chinese patients with cancer pain: a phase 3, randomized, double‐blind, multicenter study. Journal of Pain : official journal of the American Pain Society 2014;15(8):835‐44. [PUBMED: 24846822] - PubMed
References to studies excluded from this review
-
- Han HS, Lee KH, Lee KH, Ryu JS, Kim YC, Park SW, et al. A prospective, open‐label, multicenter study of the clinical efficacy of extended‐release hydromorphone in treating cancer pain inadequately controlled by other analgesics. Supportive Care in Cancer : official journal of the Multinational Association of Supportive Care in Cancer 2014;22(3):741‐50. [PUBMED: 24203087] - PubMed
-
- Lee KH, Kim MK, Hyun MS, Kim JY, Park KU, Song HS, et al. Clinical effectiveness and safety of OROS hydromorphone in breakthrough cancer pain treatment: a multicenter, prospective, open‐label study in Korean patients. Journal of Opioid Management2012; Vol. 8, issue 4:243‐52. - PubMed
-
- Wirz S, Wartenberg HC, Nadstawek J. Less nausea, emesis, and constipation comparing hydromorphone and morphine? A prospective open‐labeled investigation on cancer pain. Support Care Cancer2008; Vol. 16, issue 9:999‐1009. - PubMed
-
- Wirz S, Wittmann M, Schenk M, Schroeck A, Schaefer N, Mueller M, et al. Gastrointestinal symptoms under opioid therapy: a prospective comparison of oral sustained‐release hydromorphone, transdermal fentanyl, and transdermal buprenorphine. European Journal of Pain2009; Vol. 13, issue 7:737‐43. - PubMed
References to ongoing studies
-
- NCT02084355. Study of opioid rotation versus opioid escalation in patients with moderate to severe cancer pain [Efficacy and safety of opioid rotation compared with opioid dose escalation in patients with moderate to severe cancer pain ‐ open label, randomized, prospective study]. clinicaltrials.gov/ct2/show/NCT02084355 Date first received: 5 March 2014.
Additional references
-
- Akechi T, Okuyama T, Akizuki N, Shimizu K, Inagaki M, Fujimori M, et al. Associated and predictive factors of sleep disturbance in advanced cancer patients. Psycho‐oncology 2007;16(10):888‐94. [PUBMED: 17086580] - PubMed
-
- Ambrosio F, Paoletti F, Savoia G, Amantea B, Arcuri E, Avogaro F, et al. SIAARTI recommendations on the assessment and treatment of chronic cancer pain. Minerva Anestesiologica 2003;69(9):697‐729. [PUBMED: 14564240] - PubMed
-
- Angst MS, Drover DR, Lotsch J, Ramaswamy B, Naidu S, Wada DR, et al. Pharmacodynamics of orally administered sustained‐release hydromorphone in humans. Anesthesiology 2001;94(1):63‐73. [PUBMED: 11135723] - PubMed
-
- Bass DM, Prevo M, Waxman DS. Gastrointestinal safety of an extended‐release, nondeformable, oral dosage form (OROS): a retrospective study. Drug Safety 2002;25(14):1021‐33. [PUBMED: 12408733] - PubMed
-
- Binsfeld H, Szczepanski L, Waechter S, Richarz U, Sabatowski R. A randomized study to demonstrate noninferiority of once‐daily OROS® hydromorphone with twice‐daily sustained‐release oxycodone for moderate to severe chronic noncancer pain. Pain Practice 2010;10(5):404‐15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

