Distinct Functions of Senescence-Associated Immune Responses in Liver Tumor Surveillance and Tumor Progression
- PMID: 27728804
- PMCID: PMC7789819
- DOI: 10.1016/j.ccell.2016.09.003
Distinct Functions of Senescence-Associated Immune Responses in Liver Tumor Surveillance and Tumor Progression
Abstract
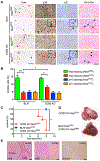
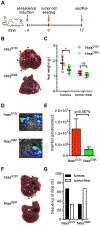
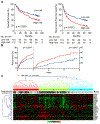
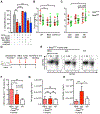
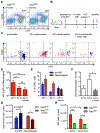
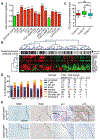
Oncogene-induced senescence causes hepatocytes to secrete cytokines, which induce their immune-mediated clearance to prevent tumor initiation, a process termed "senescence surveillance." However, senescent hepatocytes give rise to hepatocellular carcinomas (HCCs), if the senescence program is bypassed or if senescent cells are not cleared. Here, we show context-specific roles for CCR2+ myeloid cells in liver cancer. Senescence surveillance requires the recruitment and maturation of CCR2+ myeloid cells, and CCR2 ablation caused outgrowth of HCC. In contrast, HCC cells block the maturation of recruited myeloid precursors, which, through NK cell inhibition, promote growth of murine HCC and worsen the prognosis and survival of human HCC patients. Thus, while senescent hepatocyte-secreted chemokines suppress liver cancer initiation, they may accelerate the growth of fully established HCC.
Keywords: CCL2; CCR2; HCC; MDSC; NK cells; hepatocellular carcinoma; liver cancer; macrophages; myeloid cells; senescence.
Published by Elsevier Inc.
Figures

Comment in
-
Senescence and Cancer: In the Name of Immunosuppression.Cancer Cell. 2016 Oct 10;30(4):507-508. doi: 10.1016/j.ccell.2016.09.015. Cancer Cell. 2016. PMID: 27728798
Similar articles
-
Senescence surveillance of pre-malignant hepatocytes limits liver cancer development.Nature. 2011 Nov 9;479(7374):547-51. doi: 10.1038/nature10599. Nature. 2011. PMID: 22080947
-
CXCR6 Inhibits Hepatocarcinogenesis by Promoting Natural Killer T- and CD4+ T-Cell-Dependent Control of Senescence.Gastroenterology. 2019 May;156(6):1877-1889.e4. doi: 10.1053/j.gastro.2019.01.247. Epub 2019 Jan 30. Gastroenterology. 2019. PMID: 30710528
-
Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma.Gut. 2017 Jan;66(1):157-167. doi: 10.1136/gutjnl-2015-310514. Epub 2015 Oct 9. Gut. 2017. PMID: 26452628
-
Hepatocellular Senescence: Immunosurveillance and Future Senescence-Induced Therapy in Hepatocellular Carcinoma.Front Oncol. 2020 Nov 27;10:589908. doi: 10.3389/fonc.2020.589908. eCollection 2020. Front Oncol. 2020. PMID: 33330071 Free PMC article. Review.
-
In Situ Vaccination as a Strategy to Modulate the Immune Microenvironment of Hepatocellular Carcinoma.Front Immunol. 2021 May 7;12:650486. doi: 10.3389/fimmu.2021.650486. eCollection 2021. Front Immunol. 2021. PMID: 34025657 Free PMC article. Review.
Cited by
-
Senescence and the tumor-immune landscape: Implications for cancer immunotherapy.Semin Cancer Biol. 2022 Nov;86(Pt 3):827-845. doi: 10.1016/j.semcancer.2022.02.005. Epub 2022 Feb 7. Semin Cancer Biol. 2022. PMID: 35143990 Free PMC article. Review.
-
Liver, Tumor and Viral Hepatitis: Key Players in the Complex Balance Between Tolerance and Immune Activation.Front Immunol. 2020 Mar 27;11:552. doi: 10.3389/fimmu.2020.00552. eCollection 2020. Front Immunol. 2020. PMID: 32292409 Free PMC article. Review.
-
TRIM21 overexpression promotes tumor progression by regulating cell proliferation, cell migration and cell senescence in human glioma.Am J Cancer Res. 2020 Jan 1;10(1):114-130. eCollection 2020. Am J Cancer Res. 2020. PMID: 32064156 Free PMC article.
-
IER2-induced senescence drives melanoma invasion through osteopontin.Oncogene. 2021 Nov;40(47):6494-6512. doi: 10.1038/s41388-021-02027-6. Epub 2021 Oct 5. Oncogene. 2021. PMID: 34611309 Free PMC article.
-
The potential of targeting Sin3B and its associated complexes for cancer therapy.Expert Opin Ther Targets. 2017 Nov;21(11):1051-1061. doi: 10.1080/14728222.2017.1386655. Epub 2017 Oct 9. Expert Opin Ther Targets. 2017. PMID: 28956957 Free PMC article. Review.
References
-
- Acosta JC, and Gil J (2012). Senescence: a new weapon for cancer therapy. Trends in cell biology 22, 211–219. - PubMed
-
- Acosta JC, O’Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, Fumagalli M, Da Costa M, Brown C, Popov N, et al. (2008). Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 133, 1006–1018. - PubMed
-
- Cai L, Zhang Z, Zhou L, Wang H, Fu J, Zhang S, Shi M, Zhang H, Yang Y, Wu H, et al. (2008). Functional impairment in circulating and intrahepatic NK cells and relative mechanism in hepatocellular carcinoma patients. Clinical immunology 129, 428–437. - PubMed
-
- Conti I, and Rollins BJ (2004). CCL2 (monocyte chemoattractant protein-1) and cancer. Seminars in cancer biology 14, 149–154. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

