Utility of a Mouse Model of Osteoarthritis to Demonstrate Cartilage Protection by IFNγ-Primed Equine Mesenchymal Stem Cells
- PMID: 27729913
- PMCID: PMC5037129
- DOI: 10.3389/fimmu.2016.00392
Utility of a Mouse Model of Osteoarthritis to Demonstrate Cartilage Protection by IFNγ-Primed Equine Mesenchymal Stem Cells
Abstract
Objective: Mesenchymal stem cells isolated from adipose tissue (ASC) have been shown to influence the course of osteoarthritis (OA) in different animal models and are promising in veterinary medicine for horses involved in competitive sport. The aim of this study was to characterize equine ASCs (eASCs) and investigate the role of interferon-gamma (IFNγ)-priming on their therapeutic effect in a murine model of OA, which could be relevant to equine OA.
Methods: ASC were isolated from subcutaneous fat. Expression of specific markers was tested by cytometry and RT-qPCR. Differentiation potential was evaluated by histology and RT-qPCR. For functional assays, naïve or IFNγ-primed eASCs were cocultured with peripheral blood mononuclear cells or articular cartilage explants. Finally, the therapeutic effect of eASCs was tested in the model of collagenase-induced OA (CIOA) in mice.
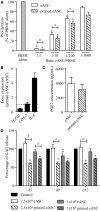
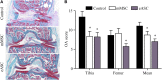
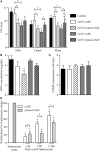
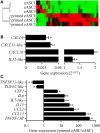
Results: The immunosuppressive function of eASCs on equine T cell proliferation and their chondroprotective effect on equine cartilage explants were demonstrated in vitro. Both cartilage degradation and T cell activation were reduced by naïve and IFNγ-primed eASCs, but IFNγ-priming enhanced these functions. In CIOA, intra-articular injection of eASCs prevented articular cartilage from degradation and IFNγ-primed eASCs were more potent than naïve cells. This effect was related to the modulation of eASC secretome by IFNγ-priming.
Conclusion: IFNγ-priming of eASCs potentiated their antiproliferative and chondroprotective functions. We demonstrated that the immunocompetent mouse model of CIOA was relevant to test the therapeutic efficacy of xenogeneic eASCs for OA and confirmed that IFNγ-primed eASCs may have a therapeutic value for musculoskeletal diseases in veterinary medicine.
Keywords: cartilage; cell therapy; horse; mesenchymal stem cells; osteoarthritis; secretome.
Figures

References
-
- Vidal MA, Kilroy GE, Lopez MJ, Johnson JR, Moore RM, Gimble JM. Characterization of equine adipose tissue-derived stromal cells: adipogenic and osteogenic capacity and comparison with bone marrow-derived mesenchymal stromal cells. Vet Surg (2007) 36(7):613–22.10.1111/j.1532-950X.2007.00313.x - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

