Introducing pharmacogenetic testing with clinical decision support into primary care: a feasibility study
- PMID: 27730116
- PMCID: PMC5047800
- DOI: 10.9778/cmajo.20150070
Introducing pharmacogenetic testing with clinical decision support into primary care: a feasibility study
Abstract
Background: Inappropriate prescribing increases patient illness and death owing to adverse drug events. The inclusion of genetic information into primary care medication practices is one solution. Our aim was to assess the ability to obtain and genotype saliva samples and to determine the levels of use of a decision support tool that creates medication options adjusted for patient characteristics, drug-drug interactions and pharmacogenetics.
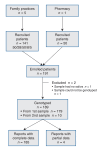
Methods: We conducted a cohort study in 6 primary care settings (5 family practices and 1 pharmacy), enrolling 191 adults with at least 1 of 10 common diseases. Saliva samples were obtained in the physician's office or pharmacy and sent to our laboratory, where DNA was extracted and genotyped and reports were generated. The reports were sent directly to the family physician/pharmacist and linked to an evidence-based prescribing decision support system. The primary outcome was ability to obtain and genotype samples. The secondary outcomes were yield and purity of DNA samples, ability to link results to decision support software and use of the decision support software.
Results: Genotyping resulted in linking of 189 patients (99%) with pharmacogenetic reports to the decision support program. A total of 96.8% of samples had at least 1 actionable genotype for medications included in the decision support system. The medication support system was used by the physicians and pharmacists 236 times over 3 months.
Interpretation: Physicians and pharmacists can collect saliva samples of sufficient quantity and quality for DNA extraction, purification and genotyping. A clinical decision support system with integrated data from pharmacogenetic tests may enable personalized prescribing within primary care. Trial registration: ClinicalTrials.gov, NCT02383290.
Conflict of interest statement
See the end of the article.
Figures
References
-
- Thomsen LA, Winterstein AG, Søndergaard B, et al. Systematic review of the incidence and characteristics of preventable adverse drug events in ambulatory care. Ann Pharmacother. 2007;41:1411–26. - PubMed
-
- Taché SV, Sönnichsen A, Ashcroft DM. Prevalence of adverse drug events in ambulatory care: a systematic review. Ann Pharmacother. 2011;45:977–89. - PubMed
-
- Gandhi TK, Weingart SN, Borus J, et al. Adverse drug events in ambulatory care. N Engl J Med. 2003;348:1556–64. - PubMed
-
- Phillips KA, Veenstra DL, Oren E, et al. Potential role of pharmacogenomics in reducing adverse drug reactions: a systematic review. JAMA. 2001;286:2270–9. - PubMed
-
- Swen JJ, Nijenhuis M, de Boer A, et al. Pharmacogenetics: from bench to byte - an update of guidelines. Clin Pharmacol Ther. 2011;89:662–73. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical