VAC chemotherapy with valproic acid for refractory/relapsing small cell lung cancer: a phase II study
- PMID: 27730152
- PMCID: PMC5005117
- DOI: 10.1183/23120541.00029-2015
VAC chemotherapy with valproic acid for refractory/relapsing small cell lung cancer: a phase II study
Abstract
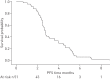
Salvage chemotherapy (CT) for relapsing or refractory small cell lung cancer (SCLC) remains disappointing. In vitro experiments showed that valproic acid increases apoptosis of SCLC cell lines exposed to doxorubicin, vindesine and bis(2-chloroethyl)amine. The primary objective of this phase II study was to determine whether epigenetic modulation with valproic acid in addition to a doxorubicin, vindesine and cyclophosphamide (VAC) regimen improves 6-month progression-free survival (PFS). Patients with pathologically proven SCLC refractory to prior platinum derivatives and etoposide were eligible. After central registration, patients received VAC plus daily oral valproic acid. 64 patients were registered, of whom six were ineligible. Seven patients did not receive any CT, leaving 51 patients assessable for the primary end-point. The objective response rate was 19.6%. Median PFS was 2.8 months (95% CI 2.5-3.6 months) and 6-month PFS was 6%. Median survival time was 5.9 months (95% CI 4.7-7.5 months). Toxicity was mainly haematological, with 88% and 26% grade 3-4 neutropenia and thrombopenia, respectively. Despite an interesting response rate, the addition of valproic acid to VAC did not translate into adequate PFS in relapsing SCLC or SCLC refractory to platinum-etoposide.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures
References
-
- Mascaux C, Paesmans M, Berghmans T, et al. . A systematic review of the role of etoposide and cisplatin in the chemotherapy of small cell lung cancer with methodology assessment and meta-analysis. Lung Cancer 2000; 30: 23–36. - PubMed
-
- O'Brien ME, Ciuleanu TE, Tsekov H, et al. . Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol 2006; 24: 5441–5447. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous