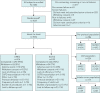
A randomised, open-label study of umeclidinium versus glycopyrronium in patients with COPD
- PMID: 27730198
- PMCID: PMC5005182
- DOI: 10.1183/23120541.00101-2015
A randomised, open-label study of umeclidinium versus glycopyrronium in patients with COPD
Abstract
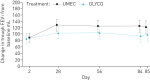
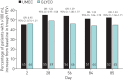
This study compared the efficacy and safety of once-daily umeclidinium 62.5 µg with once-daily glycopyrronium 50 µg in patients with moderate-to-severe chronic obstructive pulmonary disease. This was a 12-week, multicentre, randomised, open-label, parallel-group study (Clinicaltrials.gov: NCT02236611). Patients were randomised 1:1 to umeclidinium 62.5 µg or glycopyrronium 50 µg administered via Ellipta or Breezhaler dry powder inhaler, respectively. The primary endpoint was trough forced expiratory volume in 1 s (FEV1) at day 85 in the per-protocol population. Other endpoints included: weighted mean FEV1 over 0-24 h and patient-reported outcomes (transition dyspnoea index score and St George's Respiratory Questionnaire total score). Adverse events were also assessed. A total of 1037 patients were randomised to treatment. Umeclidinium was non-inferior (margin: -50 mL) to glycopyrronium (trough FEV1 at day 85 treatment difference: 24 mL, 95% confidence intervals: -5-54). Improvements in other endpoints were similar between treatments. Adverse event incidences were similar for umeclidinium (37%) and glycopyrronium (36%). Once-daily umeclidinium was non-inferior to once-daily glycopyrronium in patients with chronic obstructive pulmonary disease in trough FEV1 at day 85. Patient-reported outcomes and safety profiles were similar for both treatments.
Conflict of interest statement
can be found alongside this article at openres.ersjournals.com
Figures
References
-
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2015. www.goldcopd.org/ Date last updated: 2015. Date last accessed: August 10, 2015.
-
- Boehringer-Ingelheim. SPIRIVA. Prescribing Information. http://bidocs.boehringer-ingelheim.com/BIWebAccess/ViewServlet.ser?docBa... Date last updated: 2014. Date last accessed: September 3, 2015.
-
- Novartis. Seebri Breezhaler. Summary of product characteristics. www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information... Date last updated: 2015. Date last accessed: September 5, 2015.
-
- Novartis. Seebri Neohaler. Prescribing information. www.accessdata.fda.gov/drugsatfda_docs/label/2015/207923lbl.pdf Date last updated: 2015. Date last accessed: November 6, 2015.
-
- GlaxoSmithKline. INCRUSE. Summary of product characteristics. www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002... Date last updated: 2015. Date last accessed: September 3, 2015.
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
