The DNA cytosine deaminase APOBEC3B promotes tamoxifen resistance in ER-positive breast cancer
- PMID: 27730215
- PMCID: PMC5055383
- DOI: 10.1126/sciadv.1601737
The DNA cytosine deaminase APOBEC3B promotes tamoxifen resistance in ER-positive breast cancer
Abstract
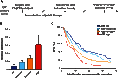
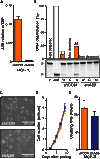
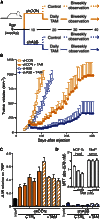
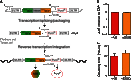
Breast tumors often display extreme genetic heterogeneity characterized by hundreds of gross chromosomal aberrations and tens of thousands of somatic mutations. Tumor evolution is thought to be ongoing and driven by multiple mutagenic processes. A major outstanding question is whether primary tumors have preexisting mutations for therapy resistance or whether additional DNA damage and mutagenesis are necessary. Drug resistance is a key measure of tumor evolvability. If a resistance mutation preexists at the time of primary tumor presentation, then the intended therapy is likely to fail. However, if resistance does not preexist, then ongoing mutational processes still have the potential to undermine therapeutic efficacy. The antiviral enzyme APOBEC3B (apolipoprotein B mRNA-editing enzyme, catalytic polypeptide-like 3B) preferentially deaminates DNA C-to-U, which results in signature C-to-T and C-to-G mutations commonly observed in breast tumors. We use clinical data and xenograft experiments to ask whether APOBEC3B contributes to ongoing breast tumor evolution and resistance to the selective estrogen receptor modulator, tamoxifen. First, APOBEC3B levels in primary estrogen receptor-positive (ER+) breast tumors inversely correlate with the clinical benefit of tamoxifen in the treatment of metastatic ER+ disease. Second, APOBEC3B depletion in an ER+ breast cancer cell line results in prolonged tamoxifen responses in murine xenograft experiments. Third, APOBEC3B overexpression accelerates the development of tamoxifen resistance in murine xenograft experiments by a mechanism that requires the enzyme's catalytic activity. These studies combine to indicate that APOBEC3B promotes drug resistance in breast cancer and that inhibiting APOBEC3B-dependent tumor evolvability may be an effective strategy to improve efficacies of targeted cancer therapies.
Keywords: APOBEC3B; DNA deamination; breast cancer; cancer mutagenesis; drug resistance; tumor evolution.
Figures
Similar articles
-
APOBEC3B is an enzymatic source of mutation in breast cancer.Nature. 2013 Feb 21;494(7437):366-70. doi: 10.1038/nature11881. Epub 2013 Feb 6. Nature. 2013. PMID: 23389445 Free PMC article.
-
Progressive APOBEC3B mRNA expression in distant breast cancer metastases.PLoS One. 2017 Jan 31;12(1):e0171343. doi: 10.1371/journal.pone.0171343. eCollection 2017. PLoS One. 2017. PMID: 28141868 Free PMC article.
-
Molecular mechanism and clinical impact of APOBEC3B-catalyzed mutagenesis in breast cancer.Breast Cancer Res. 2015 Jan 21;17(1):8. doi: 10.1186/s13058-014-0498-3. Breast Cancer Res. 2015. PMID: 25848704 Free PMC article. Review.
-
The 29.5 kb APOBEC3B Deletion Polymorphism Is Not Associated with Clinical Outcome of Breast Cancer.PLoS One. 2016 Aug 23;11(8):e0161731. doi: 10.1371/journal.pone.0161731. eCollection 2016. PLoS One. 2016. PMID: 27552096 Free PMC article.
-
APOBEC3B: pathological consequences of an innate immune DNA mutator.Biomed J. 2015 Mar-Apr;38(2):102-10. doi: 10.4103/2319-4170.148904. Biomed J. 2015. PMID: 25566802 Review.
Cited by
-
Single-cell transcriptomic profiling for inferring tumor origin and mechanisms of therapeutic resistance.NPJ Precis Oncol. 2022 Oct 10;6(1):71. doi: 10.1038/s41698-022-00314-3. NPJ Precis Oncol. 2022. PMID: 36210388 Free PMC article.
-
APOBECs and Herpesviruses.Viruses. 2021 Feb 28;13(3):390. doi: 10.3390/v13030390. Viruses. 2021. PMID: 33671095 Free PMC article. Review.
-
Acute expression of human APOBEC3B in mice results in RNA editing and lethality.Genome Biol. 2023 Nov 24;24(1):267. doi: 10.1186/s13059-023-03115-4. Genome Biol. 2023. PMID: 38001542 Free PMC article.
-
ClinOmicsTrailbc: a visual analytics tool for breast cancer treatment stratification.Bioinformatics. 2019 Dec 15;35(24):5171-5181. doi: 10.1093/bioinformatics/btz302. Bioinformatics. 2019. PMID: 31038669 Free PMC article.
-
Morphologic and Genomic Heterogeneity in the Evolution and Progression of Breast Cancer.Cancers (Basel). 2020 Mar 31;12(4):848. doi: 10.3390/cancers12040848. Cancers (Basel). 2020. PMID: 32244556 Free PMC article. Review.
References
-
- Elkin E. B., Hudis C. A., Parsing progress in breast cancer. J. Clin. Oncol. 33, 2837–2838 (2015). - PubMed
-
- Saphner T., Tormey D. C., Gray R., Annual hazard rates of recurrence for breast cancer after primary therapy. J. Clin. Oncol. 14, 2738–2746 (1996). - PubMed
-
- Burstein H. J., Temin S., Anderson H., Buchholz T. A., Davidson N. E., Gelmon K. E., Giordano S. H., Hudis C. A., Rowden D., Solky A. J., Stearns V., Winer E. P., Griggs J. J., Adjuvant endocrine therapy for women with hormone receptor–positive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update. J. Clin. Oncol. 32, 2255–2269 (2014). - PMC - PubMed
-
- Davies C., Pan H., Godwin J., Gray R., Arriagada R., Raina V., Abraham M., Medeiros Alencar V. H., Badran A., Bonfill X., Bradbury J., Clarke M., Collins R., Davis S. R., Delmestri A., Forbes J. F., Haddad P., Hou M.-F., Inbar M., Khaled H., Kielanowska J., Kwan W. H., Mathew B. S., Mittra I., Müller B., Nicolucci A., Peralta O., Pernas F., Petruzelka L., Pienkowski T., Radhika R., Rajan B., Rubach M. T., Tort S., Urrútia G., Valentini M., Wang Y., Peto R.; Adjuvant Tamoxifen: Longer Against Shorter Collaborative , Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 381, 805–816 (2013). - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

