Intrathecal Drug Delivery Systems (IDDS): The Implantable Systems Performance Registry (ISPR)
- PMID: 27730704
- PMCID: PMC5157772
- DOI: 10.1111/ner.12524
Intrathecal Drug Delivery Systems (IDDS): The Implantable Systems Performance Registry (ISPR)
Abstract
Objectives: The ISPR was initially created to monitor the product performance of Medtronic implanted intrathecal drug infusion and spinal cord systems available in the United States.
Materials and methods: Data were collected from 50 representative sites implanting and following patients with intrathecal drug delivery systems across the United States between August 7, 2003 and January 31, 2014. Device performance over time was estimated using life table survival methods.
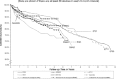
Results: Of the 6093 patients enrolled in the ISPR, 3405 (55.9%) were female and 2675 (43.9%) were male, and 13 (0.2%) did not provide gender data. The average age at enrollment was 52.9 years (SD =17.6 years) and average follow-up time was 29.6 months. Currently, the estimates of device survival from pump-related events exceed 90% for all pump models across the applicable follow-up time points. The majority of product performance events were catheter-related. At 5 years of follow-up, all applicable catheter models, with the exception of revised not as designed or grafted not as designed catheters, had greater than 81% survival from catheter-related events.
Conclusions: The ISPR is designed to serve as an ongoing source of system and device-related information with a focus on "real-world" safety and product performance. ISPR data continue to be used to guide future product development efforts aimed at improving product reliability and quality.
Keywords: intrathecal drug delivery; neuromodulation; pain; registry; spasticity.
© 2016 The Authors. Neuromodulation: Technology at the Neural Interface published by Wiley Periodicals, Inc. on behalf of International Neuromodulation Society.
Figures
References
-
- Food and Drug Administration . Development and use of risk minimization action plans. 2005. http://www.fda.gov/downloads/RegulatoryInformation/guidances/ucm126830.pdf. Accessed September 12, 2014.
-
- Food and Drug Administration . Establishing pregnancy exposure registries. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformati.... Accessed September 9, 2014.
-
- Gliklich RE, Dreyer NA. Registries for evaluating patient outcomes: a user's guide Rockville, MD: Agency for Healthcare Research and Quality, 2007. - PubMed
-
- Gliklich RE, Dreyer NA. Registries for evaluating patient outcomes: a user's guide, 2nd ed Rockville, MD: Agency for Healthcare Research and Quality, 2010. - PubMed
-
- Gliklich RE, Dreyer NA. Registries for evaluating patient outcomes: a user's guide, 3rd ed Rockville, MD: Agency for Healthcare Research and Quality, 2014. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical