Dispositional negativity: An integrative psychological and neurobiological perspective
- PMID: 27732016
- PMCID: PMC5118170
- DOI: 10.1037/bul0000073
Dispositional negativity: An integrative psychological and neurobiological perspective
Abstract
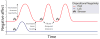
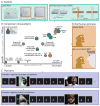
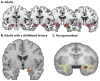
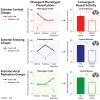
Dispositional negativity-the propensity to experience and express more frequent, intense, or enduring negative affect-is a fundamental dimension of childhood temperament and adult personality. Elevated levels of dispositional negativity can have profound consequences for health, wealth, and happiness, drawing the attention of clinicians, researchers, and policymakers. Here, we highlight recent advances in our understanding of the psychological and neurobiological processes linking stable individual differences in dispositional negativity to momentary emotional states. Self-report data suggest that 3 key pathways-increased stressor reactivity, tonic increases in negative affect, and increased stressor exposure-explain most of the heightened negative affect that characterizes individuals with a more negative disposition. Of these 3 pathways, tonically elevated, indiscriminate negative affect appears to be most central to daily life and most relevant to the development of psychopathology. New behavioral and biological data provide insights into the neural systems underlying these 3 pathways and motivate the hypothesis that seemingly "tonic" increases in negative affect may actually reflect increased reactivity to stressors that are remote, uncertain, or diffuse. Research focused on humans, monkeys, and rodents suggests that this indiscriminate negative affect reflects trait-like variation in the activity and connectivity of several key brain regions, including the central extended amygdala and parts of the prefrontal cortex. Collectively, these observations provide an integrative psychobiological framework for understanding the dynamic cascade of processes that bind emotional traits to emotional states and, ultimately, to emotional disorders and other kinds of adverse outcomes. (PsycINFO Database Record
(c) 2016 APA, all rights reserved).
Conflict of interest statement
Authors declare no conflicts of interest.
Figures

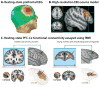
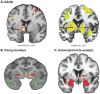
References
-
- Abercrombie HC, Schaefer SM, Larson CL, Oakes TR, Lindgren KA, Holden JE, … Davidson RJ. Metabolic rate in the right amygdala predicts negative affect in depressed patients. Neuroreport. 1998;9:3301–3307. - PubMed
-
- Ackerman RA, Corretti CA. Pathological personality traits and intimacy processes within roommate relationships. European Journal of Personality. 2015;29:152–172.
-
- Admon R, Milad MR, Hendler T. A causal model of post-traumatic stress disorder: disentangling predisposed from acquired neural abnormalities. Trends Cogn Sci. 2013;17:337–347. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

