Value of Different Comorbidity Indices for Predicting Outcome in Patients with Acute Myeloid Leukemia
- PMID: 27732646
- PMCID: PMC5061362
- DOI: 10.1371/journal.pone.0164587
Value of Different Comorbidity Indices for Predicting Outcome in Patients with Acute Myeloid Leukemia
Abstract
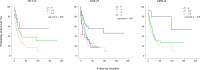
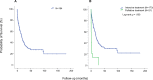
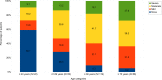
Age is a dominant predictor of outcome in acute myeloid leukemia (AML). However, it is not clear to which extent comorbidities contribute to this effect. The objective of this study was to determine the impact of pretreatment comorbidities on survival of AML patients. In a single-center retrospective study 194 adult AML patients were included. The Hematopoietic cell transplantation comorbidity index (HCT-CI), the Adult Comorbidity Evaluation-27 (ACE-27) score and the Cumulative Illness Rating Scale for Geriatrics (CIRS-G) as well as data on demographics, cytogenetics, treatment and outcome were evaluated at the time of initial diagnosis by univariate and multivariate analysis. The study included 102 male and 92 female (median age 60.9 years) of which 173 (89.2%) received intensive chemotherapy. Median overall survival (OS) was 17 months. In univariate analysis, cardiovascular disease (26 vs 12 months, p = .005), severe hepatic disease (19 vs 4 months, p = .013) and renal impairment (17 vs 7 months, p = .016) was associated with inferior OS. For each index, the highest comorbidity burden was associated with reduced OS. However, in multivariate analysis only the ACE-27 score was associated with outcome. Besides ECOG ≥ 2 and poor cytogenetics only the ACE-27 score but not higher age was associated with OS in the group of patients receiving intensive therapy. Adjusted hazard ratios were 3.1, 3.5 and 4.0 for mild, moderate and severe ACE-27-assessed comorbidities, respectively (p = .012). Our study confirms that comorbidities significantly impact survival of AML patients and a pretreatment assessment of comorbidities may help to identify patients with poor outcome.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Röllig C, Bornhäuser M, Thiede C, Taube F, Kramer M, Mohr B, et al. Long-term prognosis of acute myeloid leukemia according to the new genetic risk classification of the European LeukemiaNet recommendations: evaluation of the proposed reporting system. J Clin Oncol 2011; 29(20): 2758–65. 10.1200/JCO.2010.32.8500 - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

