A Phase II Clinical Trial of CPI-613 in Patients with Relapsed or Refractory Small Cell Lung Carcinoma
- PMID: 27732654
- PMCID: PMC5061374
- DOI: 10.1371/journal.pone.0164244
A Phase II Clinical Trial of CPI-613 in Patients with Relapsed or Refractory Small Cell Lung Carcinoma
Abstract
Background: Small cell lung cancer (SCLC) is a common lung cancer which presents with extensive stage disease at time of diagnosis in two-thirds of patients. For treatment of advanced disease, traditional platinum doublet chemotherapy induces response rates up to 80% but with few durable responses. CPI-613 is a novel anti-cancer agent that selectively inhibits the altered form of mitochondrial energy metabolism in tumor cells.
Methods: We evaluated CPI-613 with a single-arm, open-label phase II study in patients with relapsed or refractory SCLC. CPI-613 was given at a dose of 3,000 mg/m2 on days 1 and 4 of weeks 1-3 of 4 week cycle. The primary outcome was response rate as assessed by CT imaging using RECIST v1.1 criteria. Secondary outcomes were progression-free survival (PFS), overall survival (OS), and toxicity. Twelve patients were accrued (median age 57yo) who had previously received between 1 and 4 lines of chemotherapy (median 1) for SCLC with a treatment-free interval of less than 60 days in 9 of the 12 patients.
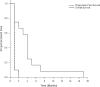
Results: No complete or partial responses were seen. Ten patients (83%) progressed as best response and 2 (17%) were not evaluable for response. Median time to progression was 1.7 months (range 0.7 to 1.8 months). Eleven patients (92%) died with median overall survival of 4.3 months (range 1.2 to 18.2 months). The study was closed early due to lack of efficacy. Of note, three out of three patients who progressed after CPI-613 and were subsequently treated with standard topotecan then demonstrated treatment response with survival for 18.2, 7.4, and 5.1 months. We conducted laboratory studies which found synergy in-vitro for CPI-613 with topotecan.
Conclusions: Single agent CPI-613 had no efficacy in this study. Further study of CPI 613 in combination with a topoisomerase inhibitor is warranted.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Smith IE, Evans BD, Gore ME, Vincent MD, Repetto L, Yarnold JR, et al. Carboplatin (Paraplatin; JM8) and etoposide (VP-16) as first-line combination therapy for small-cell lung cancer. J Clin Oncol. 1987. February;5(2):185–9. - PubMed
-
- O’Brien MER, Ciuleanu T-E, Tsekov H, Shparyk Y, Cuceviá B, Juhasz G, et al. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol. 2006. December 1;24(34):5441–7. 10.1200/JCO.2006.06.5821 - DOI - PubMed
-
- Pardee TS, Lee K, Luddy J, Maturo C, Rodriguez R, Isom S, et al. A phase I study of the first-in-class antimitochondrial metabolism agent, CPI-613, in patients with advanced hematologic malignancies. Clin Cancer Res. 2014. October 15;20(20):5255–64. 10.1158/1078-0432.CCR-14-1019 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

