NAD+ Replenishment Improves Lifespan and Healthspan in Ataxia Telangiectasia Models via Mitophagy and DNA Repair
- PMID: 27732836
- PMCID: PMC5777858
- DOI: 10.1016/j.cmet.2016.09.004
NAD+ Replenishment Improves Lifespan and Healthspan in Ataxia Telangiectasia Models via Mitophagy and DNA Repair
Abstract
Ataxia telangiectasia (A-T) is a rare autosomal recessive disease characterized by progressive neurodegeneration and cerebellar ataxia. A-T is causally linked to defects in ATM, a master regulator of the response to and repair of DNA double-strand breaks. The molecular basis of cerebellar atrophy and neurodegeneration in A-T patients is unclear. Here we report and examine the significance of increased PARylation, low NAD+, and mitochondrial dysfunction in ATM-deficient neurons, mice, and worms. Treatments that replenish intracellular NAD+ reduce the severity of A-T neuropathology, normalize neuromuscular function, delay memory loss, and extend lifespan in both animal models. Mechanistically, treatments that increase intracellular NAD+ also stimulate neuronal DNA repair and improve mitochondrial quality via mitophagy. This work links two major theories on aging, DNA damage accumulation, and mitochondrial dysfunction through nuclear DNA damage-induced nuclear-mitochondrial signaling, and demonstrates that they are important pathophysiological determinants in premature aging of A-T, pointing to therapeutic interventions.
Published by Elsevier Inc.
Conflict of interest statement
The Bohr laboratory has CRADA arrangements with ChromaDex and GlaxoSmithKline. DAS is a consultant for GSK, Ovascience and Metrobiotech.
Figures
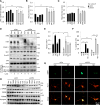
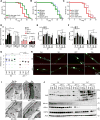

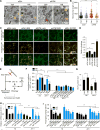



Comment in
-
Overcoming ATM Deficiency by Activating the NAD+/SIRT1 Axis.Cell Metab. 2016 Oct 11;24(4):526-528. doi: 10.1016/j.cmet.2016.09.019. Cell Metab. 2016. PMID: 27732834
References
-
- Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. - PubMed
-
- Barlow C, Hirotsune S, Paylor R, Liyanage M, Eckhaus M, Collins F, Shiloh Y, Crawley JN, Ried T, Tagle D, et al. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell. 1996;86:159–171. - PubMed
-
- Berdichevsky A, Viswanathan M, Horvitz HR, Guarente L. C. elegans SIR-2.1 interacts with 14-3-3 proteins to activate DAF-16 and extend life span. Cell. 2006;125:1165–1177. - PubMed
-
- Bieganowski P, Brenner C. Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss- Handler independent route to NAD+ in fungi and humans. Cell. 2004;117:495–502. - PubMed
-
- Canto C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, Fernandez-Marcos PJ, Yamamoto H, Andreux PA, Cettour-Rose P, et al. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell metabolism. 2012;15:838–847. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

