Improved outcome in acute myeloid leukemia patients enrolled in clinical trials: A national population-based cohort study of Danish intensive chemotherapy patients
- PMID: 27732947
- PMCID: PMC5342143
- DOI: 10.18632/oncotarget.12495
Improved outcome in acute myeloid leukemia patients enrolled in clinical trials: A national population-based cohort study of Danish intensive chemotherapy patients
Abstract
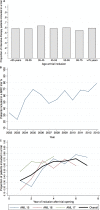
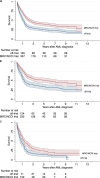
Clinical trials are critical to improve AML treatment. It remains, however, unclear if clinical trial participation per se affects prognosis and to what extent the patients selected for trials differ from those of patients receiving intensive therapy off-trial.We conducted a population-based cohort study of newly diagnosed Danish AML patients treated with intensive chemotherapy between 2000-2013. We estimated accrual rates and compared characteristics, complete remission (CR) rates, and relative risks (RRs) of death at 90-day, 1-year, and 3-years in clinical trial patients to patients treated off-trial.Of 867 patients, 58.3% (n = 504) were included in a clinical trial. Accrual rates were similar across age groups (p = 0.55). Patients with poor performance status, comorbidity, therapy-related and secondary AML were less likely to be enrolled in trials. CR rates were 80.2% in trial-patients versus 68.6% in patients treated off- trial. Also, trial-patients had superior survival at 1-year; 72%, vs. 54% (adjusted RR of death 1.28(CI = 1.06-1.54)), and at 3 years; 45% vs. 29% (adjusted RR 1.14(CI = 1.03-1.26)) compared to patients treated off-trial.Despite high accrual rates, patients enrolled in clinical trials had a favorable prognostic profile and a better survival than patients treated off-trial. In conclusion, all trial results should be extrapolated with caution and population-based studies of "real world patients" have a prominent role in examining the prognosis of AML.
Keywords: acute myeloid leukemia; chemotherapy; population-based; prognosis; trials.
Conflict of interest statement
The authors declare that there are no conflicts of interest to report.
Figures
References
-
- Mengis C, Aebi S, Tobler A, Dahler W, Fey MF. Assessment of differences in patient populations selected for excluded from participation in clinical phase III acute myelogenous leukemia trials. Journal of clinical oncology. 2003;21:3933–3939. - PubMed
-
- Fern LA, Lewandowski JA, Coxon KM, Whelan J, for the National Cancer Research Institute Teenage and Young Adult Clinical Studies Group, UK Available, accessible, aware, appropriate, and acceptable: a strategy to improve participation of teenagers and young adults in cancer trials. The lancet oncology. 2014;15:e341–e350. - PubMed
-
- Sateren WB, Trimble EL, Abrams J, Brawley O, Breen N, Ford L, McCabe M, Kaplan R, Smith M, Ungerleider R, Christian MC. How sociodemographics, presence of oncology specialists, and hospital cancer programs affect accrual to cancer treatment trials. Journal of clinical oncology. 2002;20:2109–2117. - PubMed
-
- Ford JG, Howerton MW, Lai GY, Gary TL, Bolen S, Gibbons MC, Tilburt J, Baffi C, Tanpitukpongse TP, Wilson RF, Powe NR, Bass EB. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 2008;112:228–242. - PubMed
-
- Dechartres A, Chevret S, Lambert J, Calvo F, Levy V. Inclusion of patients with acute leukemia in clinical trials: a prospective multicenter survey of 1066 cases. Annals of Oncology/ESMO. 2011;22:224–233. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

