Cerium Oxide Nanoparticles Improve Outcome after In Vitro and In Vivo Mild Traumatic Brain Injury
- PMID: 27733104
- PMCID: PMC7249477
- DOI: 10.1089/neu.2016.4644
Cerium Oxide Nanoparticles Improve Outcome after In Vitro and In Vivo Mild Traumatic Brain Injury
Abstract
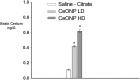
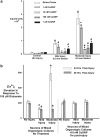
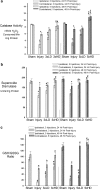
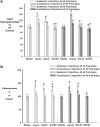
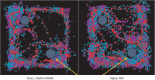
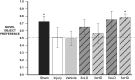
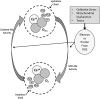
Mild traumatic brain injury results in aberrant free radical generation, which is associated with oxidative stress, secondary injury signaling cascades, mitochondrial dysfunction, and poor functional outcome. Pharmacological targeting of free radicals with antioxidants has been examined as an approach to treatment, but has met with limited success in clinical trials. Conventional antioxidants that are currently available scavenge a single free radical before they are destroyed in the process. Here, we report for the first time that a novel regenerative cerium oxide nanoparticle antioxidant reduces neuronal death and calcium dysregulation after in vitro trauma. Further, using an in vivo model of mild lateral fluid percussion brain injury in the rat, we report that cerium oxide nanoparticles also preserve endogenous antioxidant systems, decrease macromolecular free radical damage, and improve cognitive function. Taken together, our results demonstrate that cerium oxide nanoparticles are a novel nanopharmaceutical with potential for mitigating neuropathological effects of mild traumatic brain injury and modifying the course of recovery.
Keywords: cerium oxide; nanoparticles; oxidative stress; traumatic brain injury.
Conflict of interest statement
No competing financial interests exist.
Figures

References
-
- Smith S.L., Andrus P.K., Zhang J.R., and Hall E.D. (1994). Direct measurement of hydroxyl radicals, lipid peroxidation, and blood–brain barrier disruption following unilateral cortical impact head injury in the rat. J. Neurotrauma 11, 393–404 - PubMed
-
- Kontos H.A., and Wei E.P. (1986). Superoxide production in experimental brain injury. J. Neurosurg. 64, 803–807 - PubMed
-
- Lafon–Cazal M., Pietri S., Culcasi M., and Bockaert J. (1993). NMDA-dependent superoxide production and neurotoxicity. Nature 364, 535–537 - PubMed
-
- Globus M.Y., Alonso O., Dietrich W.D., Busto R., and Ginsberg M.D. (1995). Glutamate release and free radical production following brain injury: effects of posttraumatic hypothermia. J. Neurochem. 65, 1704–1711 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
